
Concept explainers
Interpretation:
The name and structure of the alkyl group corresponding to methane should be determined.
Concept introduction:
The group formed by the loss of hydrogen atom from the corresponding
Answer to Problem 52A
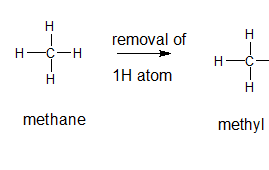
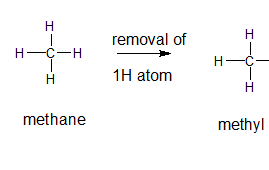
Explanation of Solution
A type of functional group that contains only hydrogen and carbon atoms in its structure is said to be alkyl group and are formed by the loss of hydrogen atom from the corresponding alkane. In order to name the alkyl groups, the suffix -ane of the alkane are dropped and the suffix -yl is added to name that is the alkane name is changed to alkyl.
The general formula for an alkane group is
The given parent alkane is methane, 3
Interpretation:
The name and structure of the alkyl group corresponding to butane should be determined.
Concept introduction:
The group formed by the loss of hydrogen atom from the corresponding alkane is said to be the alkyl group. In order to name the alkyl groups, the suffix -ane of the alkane are dropped and the suffix -yl is added to name that is the alkane name is changed to alkyl.
Answer to Problem 52A
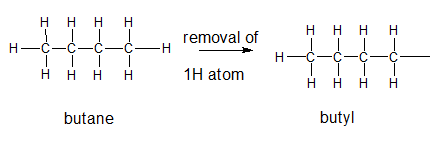
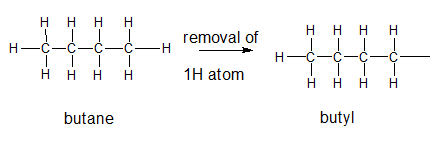
Explanation of Solution
The general formula for an alkane group is
The given parent alkane is butane,
Interpretation:
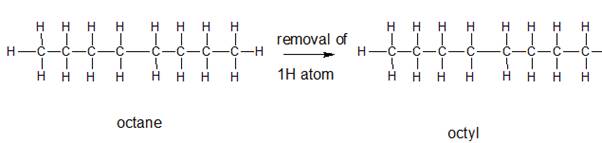
The name and structure of the alkyl group corresponding to octane should be determined.
Concept introduction:
The group formed by the loss of hydrogen atom from the corresponding alkane is said to be the alkyl group. In order to name the alkyl groups, the suffix -ane of the alkane are dropped and the suffix -yl is added to name that is the alkane name is changed to alkyl.
Answer to Problem 52A
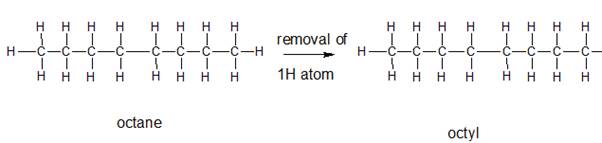
Explanation of Solution
The general formula for an alkane group is
The given parent alkane is octane,
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: The Central Science (14th Edition)
Chemistry: The Central Science (13th Edition)
Organic Chemistry (9th Edition)
Inorganic Chemistry
Chemistry: A Molecular Approach
Chemistry: A Molecular Approach (4th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





