
Concept explainers
(a)
Interpretation:
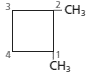
Whether the numbering of following structure is correct or not needs to be determined and if the numbering is wrong then the structure needs to be redrawn with correct numbering:
Concept introduction:
The hydrocarbon compounds contain compound that are made up of only hydrogen and carbon atoms.
The naming of the hydrocarbon compounds is done in such a way that substituents gets lower number.
(a)
Answer to Problem 83A
The numbering for the given compound is correct.
Explanation of Solution
The given compound is disubstituted cycloalkane, so the main group is cycloalkane since, the cycloalkane is made up of four carbon atoms so, it is named as cyclobutane and the numbering of the two methyl substituents is done in such a way that it gets lower number that is 1 and 2 so, the given numbering is correct and name of the compound is 1, 2-dimethylcyclobutane.
(b)
Interpretation:
Whether the numbering of following structure is correct or not needs to be determined and if the numbering is wrong then the structure needs to be redrawn with correct numbering:
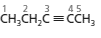
Concept introduction:
The hydrocarbon compounds contain compound that are made up of only hydrogen and carbon atoms.
The naming of the hydrocarbon compounds is done in such a way that substituents gets lower number.
(b)
Answer to Problem 83A
The numbering for the given compound is incorrect. The correct numbering is:
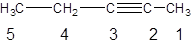
Explanation of Solution
The given compound is
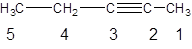
Hence, the given numbering is incorrect and name of the compound is pent-2-yne.
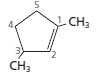
(c)
Interpretation:
Whether the numbering of following structure is correct or not needs to be determined and if the numbering is wrong then the structure needs to be redrawn with correct numbering:
Concept introduction:
The hydrocarbon compounds contain compound that are made up of only hydrogen and carbon atoms.
The naming of the hydrocarbon compounds is done in such a way that substituents gets lower number.
(c)
Answer to Problem 83A
The numbering for the given compound is correct.
Explanation of Solution
The given compound is disubstituted cycloalkene, so the main group is cycloalkene since, the cycloalkene is made up of five carbon atoms so, it is named as cyclopentene and the numbering of the two methyl substituents is done in such a way that double bond gets the lowest possible number. Hence, the given numbering is correct and name of the compound is 1, 3-dimethylcyclopent-1-ene.
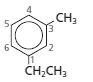
(d)
Interpretation:
Whether the numbering of following structure is correct or not needs to be determined and if the numbering is wrong then the structure needs to be redrawn with correct numbering:
Concept introduction:
The hydrocarbon compounds contain compound that are made up of only hydrogen and carbon atoms.
The naming of the hydrocarbon compounds is done in such a way that substituents gets lower number.
(d)
Answer to Problem 83A
The numbering for the given compound is correct.
Explanation of Solution
The given compound is disubstituted benzene, so the main group is benzene. The numbering of the two substituents that is ethyl and methyl is done in such a way it gets the lowest possible number. Hence, the given numbering is correct and name of the compound is written in alphabetical order as 1-ethyl-3-methylbenzene.
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Introductory Chemistry (6th Edition)
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry: The Central Science (13th Edition)
Organic Chemistry (8th Edition)
Essential Organic Chemistry (3rd Edition)
Chemistry: The Central Science (14th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





