
Interpretation:
The information given in the table needs to be drawn in graph and then the boiling and melting point of 11- and 12-carbon
Concept introduction:
The temperature at which a liquid substance becomes gas is said to be boiling point. At this temperature, the pressure surrounding the liquid and vapor pressure of the liquid becomes equal.
The temperature at which a solid substance becomes liquid is said to be melting point. At this temperature, the solid and liquid phases exist in equilibrium.
Explanation of Solution
Given:
The table:
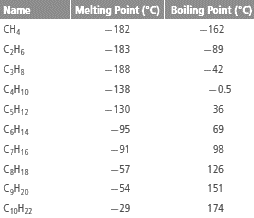
From the given table, it can be observed that with increase in number of C atoms in alkane, the melting and boiling point increases.
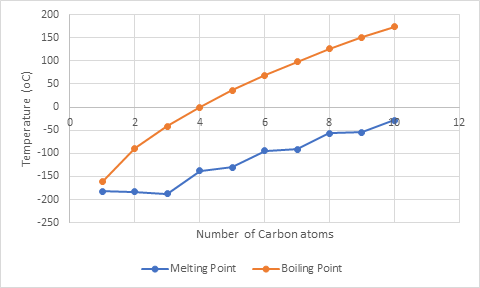
The graph between melting and boiling point and number of C atoms in alkane where y-axis represents temperature and x-axis represents number of C atoms in alkane is:
Now, the boiling and melting point of 11- and 12-carbon alkanes can be predicted by extrapolating the best fit line as:
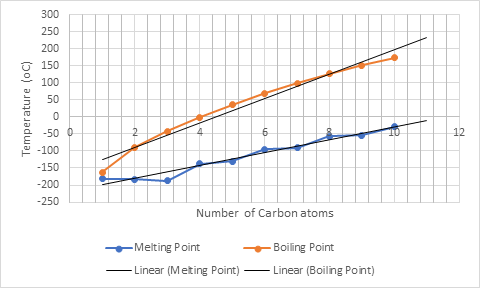
For C-11 boiling point and melting point is approximately
For C-12 boiling point and melting point is approximately
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: A Molecular Approach
Chemistry: The Central Science (13th Edition)
Introductory Chemistry (6th Edition)
Organic Chemistry (8th Edition)
Essential Organic Chemistry (3rd Edition)
Chemistry: Structure and Properties
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





