
Concept explainers
a.
Interpretation:
The name of the given
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In case of amine, suffix amine is added to the name of the carbon chain. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
a.
Explanation of Solution
The condensed formula of given amine is as follows:
The structural formula for the same can be drawn as follows:
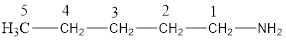
From the above structural formula, it is clear that there are 5 C atoms in the main chain.
Also, there is an amine group at 1st position therefore; the name of the amine is pentan-1-amine.
b.
Interpretation:
The name of the given amine needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In case of amine, suffix amine is added to the name of the carbon chain. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
b.
Explanation of Solution
The condensed formula of given amine is as follows:
The structural formula for the same can be drawn as follows:
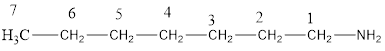
From the above structural formula, it is clear that there are 7 C atoms in the main chain.
Also, there is an amine group at 1st position therefore; the name of the amine is heptan-1-amine.
c.
Interpretation:
The name of the given amine needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In case of amine, suffix amine is added to the name of the carbon chain. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
c.
Explanation of Solution
The condensed formula of given amine is as follows:
The structural formula for the same can be drawn as follows:
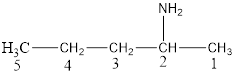
From the above structural formula, it is clear that there are 5 C atoms in the main chain.
Also, there is an amine group at 2nd position therefore; the name of the amine is pentan-2-amine.
d.
Interpretation:
The name of the given amine needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In case of amine, suffix amine is added to the name of the carbon chain. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
d.
Explanation of Solution
The condensed formula of given amine is as follows:
The structural formula for the same can be drawn as follows:
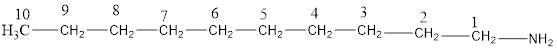
From the above structural formula, it is clear that there are 10 C atoms in the main chain.
Also, there is an amine group at 1st position therefore; the name of the amine is decan-1-amine.
Chapter 22 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: Structure and Properties
General Chemistry: Principles and Modern Applications (11th Edition)
Inorganic Chemistry
Chemistry: A Molecular Approach
Organic Chemistry (9th Edition)
Introductory Chemistry (6th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





