
(a)
Interpretation:
The type of given carbonyl compound needs to be determined.
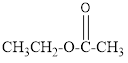
Concept introduction:
The functional group is the group present on the main carbon chain of an organic compound. It determines the chemical properties and the type of reactions an organic compound can show. The name of the group depends on the atoms present in the group for example an −OH group contains O and H atom thus, it is a hydroxyl group. The different
Name of some functional groups are: -OH: alcohol, -CHO:
Answer to Problem 14SSC
Ester.
Explanation of Solution
The given organic compound is as follows:
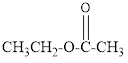
In the above organic compound, the general formula is R-COOR’ thus, it is an ester.
(b)
Interpretation:
The type of given carbonyl compound needs to be determined.
Concept introduction:
The functional group is the group present on the main carbon chain of an organic compound. It determines the chemical properties and the type of reactions an organic compound can show. The name of the group depends on the atoms present in the group for example an −OH group contains O and H atom thus, it is a hydroxyl group. The different functional groups are named according to IUPAC rule.
Name of some functional groups are: -OH: alcohol, -CHO: aldehyde, -COOH: carboxylic acid etc.
Answer to Problem 14SSC
Explanation of Solution
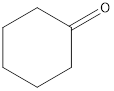
The given organic compound is as follows:
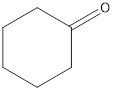
In the above organic compound, the functional group is −CO- which is a ketone group. Thus, it is a ketone.
(c)
Interpretation:
The type of given carbonyl compound needs to be determined.

Concept introduction:
The functional group is the group present on the main carbon chain of an organic compound. It determines the chemical properties and the type of reactions an organic compound can show. The name of the group depends on the atoms present in the group for example an −OH group contains O and H atom thus, it is a hydroxyl group. The different functional groups are named according to IUPAC rule.
Name of some functional groups are: -OH: alcohol, -CHO: aldehyde, -COOH: carboxylic acid etc.
Answer to Problem 14SSC
Amide.
Explanation of Solution
The given organic compound is as follows:

In the above organic compound, the general formula is RCONH2. Here, -CONH2 is an amide functional group thus, it is an amide.
(d)
Interpretation:
The type of given carbonyl compound needs to be determined.
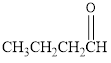
Concept introduction:
The functional group is the group present on the main carbon chain of an organic compound. It determines the chemical properties and the type of reactions an organic compound can show. The name of the group depends on the atoms present in the group for example an −OH group contains O and H atom thus, it is a hydroxyl group. The different functional groups are named according to IUPAC rule.
Name of some functional groups are: -OH: alcohol, -CHO: aldehyde, -COOH: carboxylic acid etc.
Answer to Problem 14SSC
Aldehyde.
Explanation of Solution
The given organic compound is as follows:
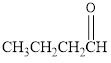
In the above organic compound, the general formula is R-CHO, here, -CHO is an aldehyde functional group thus, it is an aldehyde.
Chapter 22 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Organic Chemistry
Chemistry: A Molecular Approach (4th Edition)
Introductory Chemistry (6th Edition)
Organic Chemistry (8th Edition)
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry: The Central Science (14th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





