
(a)
Interpretation:
The structure and name of the organic compound formed by reaction of ethene and water need to be determined.
Concept introduction:
In a substitution reaction, one functional group is replaced by other functional group. In addition reaction, two reactants simply add to form a single product. In condensation reaction, addition of two reactants takes place after removal of water molecule. The elimination reaction results when removal of two substituent groups takes place from a molecule.
Explanation of Solution
The addition of water to
For example:
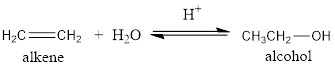
Thus, the alcohol formed from ethane will be ethanol.
(b)
Interpretation:
The structure and name of the organic compound formed by reaction of ethene and hydrogen need to be determined.
Concept introduction:
In a substitution reaction, one functional group is replaced by other functional group. In addition reaction, two reactants simply add to form a single product. In condensation reaction, addition of two reactants takes place after removal of water molecule. The elimination reaction results when removal of two substituent groups takes place from a molecule.
Explanation of Solution
Ethene can reacts with hydrogen gas to form ethane. This result in simple addition of H atom each on the two C atoms joined via a double bond.
The reaction is reduction of ethane to ethane and this is represented as follows:
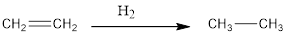
(c)
Interpretation:
The structure and name of the organic compound formed by reaction of ethene and hydrogen chloride need to be determined.
Concept introduction:
In a substitution reaction, one functional group is replaced by other functional group. In addition reaction, two reactants simply add to form a single product. In condensation reaction, addition of two reactants takes place after removal of water molecule. The elimination reaction results when removal of two substituent groups takes place from a molecule.
Explanation of Solution
The addition reaction of hydrogen chloride to alkene results in the formation of
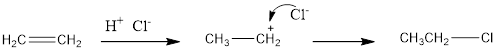
Thus, the reaction results in the formation of ethyl chloride.
(d)
Interpretation:
The structure and name of the organic compound formed by reaction of ethene and fluorine need to be determined.
Concept introduction:
In a substitution reaction, one functional group is replaced by other functional group. In addition reaction, two reactants simply add to form a single product. In condensation reaction, addition of two reactants takes place after removal of water molecule. The elimination reaction results when removal of two substituent groups takes place from a molecule.
Explanation of Solution
Ethene reacts with fluorine to form carbon and hydrogen fluoride.
The reaction of fluorine gas is very explosive in nature. The reaction is represented as follows:

Chapter 22 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Introductory Chemistry (6th Edition)
Organic Chemistry
Chemistry: Structure and Properties
General Chemistry: Principles and Modern Applications (11th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
Essential Organic Chemistry (3rd Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





