
Interpretation:
The reason for ethanol to have higher boiling point than aminoethane needs to be determined.
Concept introduction:
The boiling point of an organic compound depends on the structure of the compound and its molecular mass. Intermolecular forces in organic compounds also play an important role in determining the boiling point.
Explanation of Solution
The three important factors on which the boiling point of an organic compound depends are as follows:
- Intermolecular forces such as hydrogen bonding, ionic, dipole-dipole and Van der Waals forces.
- If number of carbon atoms in an organic compound increases, boiling point also increases.
- If in an organic compound branching increases, boiling point will decrease.
The structural formulas of ethanol and aminoethane are as follows:
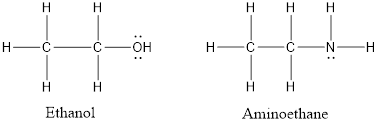
Here, O is more electronegative than N atom thus, the hydrogen bonding formed in the case of ethanol will be stronger than aminoethane. Also, due to higher electronegtaivity of O atom the dipole-dipole interactions in case of ethanol molecules will be more. This results in higher boiling point of ethanol than aminoethane.
Chapter 22 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
CHEMISTRY-TEXT
Chemistry: A Molecular Approach (4th Edition)
Chemistry: Structure and Properties (2nd Edition)
Chemistry: Structure and Properties
Introductory Chemistry (6th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





