
Concept explainers
(a)
Interpretation:
The given amino acid is to be prepared using acetoamidomalonate method.
Concept Introduction:
The method used for preparation of
Answer:
The synthesis of given amino acid using acetoamidomalonate method is shown below.
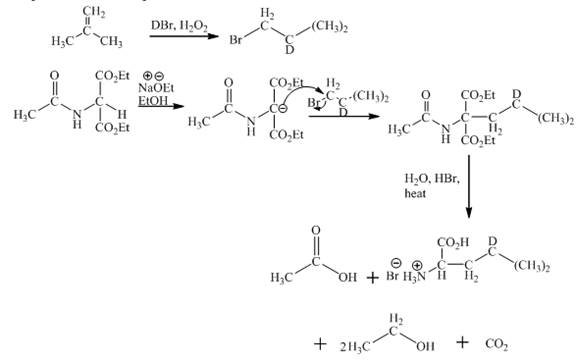
Explanation:
The given isobutylene reacts with DBr/H2O to form bromoisobutane. Diethylacetamidomalonate forms enolate ion in the presence of sodium ethoxide and ethanol. Then enolate ion is alkylated with bromoisobutane. The compound formed is treated with hot aqueous hydrogen bromide. First, the ester hydrolysis takes place. Second, the decarboxylation of carboxylic group takes place. Third, acetamido group and amide gets hydrolysed. This results in the product formation. The complete reaction is shown below.
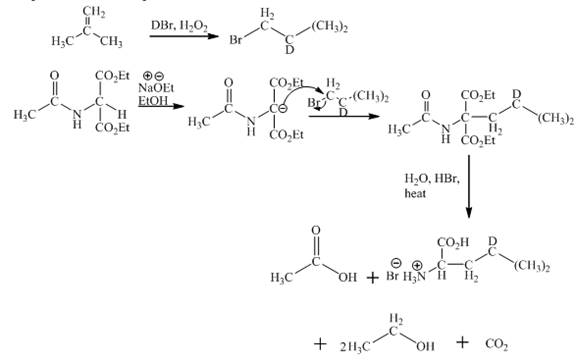 Figure 1
Figure 1
Conclusion:
The complete synthesis of given compound is shown in Figure 1.
(b)
Interpretation:
The given amino acid is to be prepared using acetoamidomalonate method.
Concept Introduction:
The method used for preparation of
(c)
Interpretation:
The given amino acid is to be prepared using acetoamidomalonate method.
Concept Introduction:
The method used for preparation of
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
EBK ORGANIC CHEMISTRY
- 4(a) Suggest a test you will use to show that a given food substance contains protein.(b)Show how you will use(i)Modified Gabriel’s Synthsis(ii)Streckers’s Synthesis to prepare phenylalanine in the laboratory.arrow_forwardWhen aspirin is made from acetic acid, the by product is H2O; when acetic anhydride 1Sused, the by-product is acetic acid. When acetyl chloride is used (see above), the by product is HC1. Which route should you use if you were working for Leica, a company that mass produces aspirin on a daily basis? Why?arrow_forwardPredict the products obtained from the reaction of triolein with the following reagents.(a) NaOH in water (b) H2 and a nickel catalystarrow_forward
- a) Explain why the acetamido group is an ortho, para-directing group. Why should it be less effective in activating the aromatic ring toward further substitution than an amino group? b) o-Nitroaniline is more soluble in ethanol than p-nitroaniline. Propose a flow scheme by which a pure sample of o-nitroaniline might be obtained from this reaction.arrow_forwardSuggest a test you will use to show that a given food substance contains protein. Show how you will use; A modified Gabriel's synthesis A Streckers' synthesis to prepare phenylalanine in the laboratory.arrow_forwardWhich group is a stronger activator between the amino group of aniline and the acetamido group of acetanilide? Because?arrow_forward
- The pKa of the conjugate acid of guanidine is 13.6, making it one of thestrongest neutral organic bases. Offer an explanation.arrow_forward-Amino acids can be prepared by the Strecker synthesis, a two-step process in which an aldehyde is treated with ammonium cyanide followed by hydrolysis of the amino nitrile intermediate with aqueous acid. Propose a mechanism for the reaction.arrow_forward26. (i) Why is the ester formation of alcohols and acid chlorides done in a weakly basic solution like pyrmidine? (ii) Why is this setup not viable for alcohols and carboxylic acids to form esters? pyrimidine deprotonates the alcohol; the alkoxide ion forms pyrimidine deprotonates the alcohol; the carboxylate ion forms pyrimidine deprotonates the tetrahedral intermediate; the alkoxide ion forms pyrimidine deprotonates the tetrahedral intermediate; the carboxylate ion formsarrow_forward
- Predict the products obtained from the reaction of triolein with the following reagents.(a) NaOH in waterarrow_forwardCompounds with polyfunctional groups must follow priority rule. Which of the following compound is correctly named. Justify your claim and construct its correct structure. Compound A: 3-amino-7-carboxy-4-oxoheptanamide Compound B: 3-amino-7-carbamoyl-4-oxoheptanoic acid Compound C: 3-amino-4-ox0-7-carbamoylheptanoic acid Compound D: 3-amino-4-oxo-7-carboxyheptanamidearrow_forwardThe reaction of an ester with an amine is not as slow as the reaction of an ester with water or an alcohol. Explain with reason. Explain why the rate of aminolysis of an ester cannot be increased by H+, OH- or OR-. How can you activate the carboxylic acid? Is acid catalyzed hydrolysis of acetamide a reversible or an irreversible reaction. Explain.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

