
Concept explainers
For each
Interpretation:
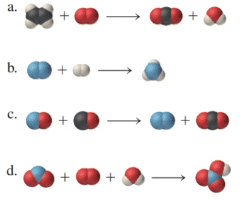
The chemical reactions are to be balanced for the given molecular model reactions, where carbon atoms are black, hydrogen atoms are white, oxygen atoms are red, and nitrogen atoms are blue.
Concept Introduction:
A chemical equation is considered to be the symbolic depiction of a chemical reaction, which contains symbols and formula.
In a chemical reaction, the starting substances on the left side are known as reactants, while the substances on the right side are known as products.
The chemical formula represents the precise number of atoms in the molecule, but not their structural arrangement
The molecular model represents the molecules in a three-dimensional configuration.
Answer to Problem 63E
Solution: The chemical formulas are represented as follows: a)
Explanation of Solution
a)
In the given molecular model, the reaction can be represented in a chemical reaction as follows:
In the next step, the
Now, in the final step, the
b)
In the given molecular model, the reaction can be represented in a chemical reaction as follows:
In the next step, the
c)
In the given molecular model, the reaction can be represented in a chemical reaction as follows:
In next step, the
Now, in the final step, the
d)
In the given molecular model, the reaction can be represented in a chemical reaction as follows:
In the next step,
Now, in the final step, the
Want to see more full solutions like this?
Chapter 4 Solutions
Chemistry In Focus
- The age of the universe is unknown, but some conclude from measuring Hubbles constant that the age is about 18 billion years old, which is about four times the age of Earth. If so, calculate the age of the universe in seconds. If you had a sample of carbon with the same number of carbon atoms as there have been seconds since the universe began, determine whether you could measure this sample on a laboratory balance that can detect masses as small as 0.1 mg.arrow_forwardBalance the following equations by filling in the blanks. (a) 92235U+01n54137_+201n+_ (b) 90232Th+612__01n+96240Cm (c) 24He+4296Mo43100_+_ (d) _+12H84210_+01narrow_forwardName and describe the composition of the three hydrogen isotopes.arrow_forward
- Reference Section 5-2 to find the atomic masses of 12C and 13C, the relative abundance of 12C and 13C in natural carbon, and the average mass (in u) of a carbon atom. If you had a sample of natural carbon containing exactly 10,000 atoms, determine the number of 12C and 13C atoms present. What would be the average mass (in u) and the total mass (in u) of the carbon atoms in this 10,000-atom sample? If you had a sample of natural carbon containing 6.0221 1023 atoms, determine the number of 12C and 13C atoms present What would be the average mass (in u) and the total mass (in u) of this 6.0221 1023 atom sample? Given that 1 g = 6.0221 1023 u, what is the total mass of I mole of natural carbon in units of grams?arrow_forwardAn adult human body contains 6.0 L blood, which contains about 15.5 g hemoglobin per 100.0 mL blood. The molar mass of hemoglobin is approximately 64,500 g/mol and there is 4 mol iron per 1 mol hemoglobin. A news item claims that there is sufficient iron in the hemoglobin of the body that this iron, if it were in the form of metallic iron, could make a 3-in. iron nail that weighs approximately 3.7 g. Show sufficient calculations to either support or refute the claim.arrow_forwardWhat is the chemical formula for each substance mentioned? (A). Sodium hydrogen carbonate (B). Calcium hypochlorite (C). Hydrogen cyanide (D). Magnesium hydroxide (E). Tin(II) fluoride (F). cadmium sulfidearrow_forward
- Use triangles to represent atoms of element A and circles to represent atoms of element B. Draw an atomic level view of a homogeneous mixture of elements A and B. Draw an atomic view of the compound AB in a liquid state (molecules close together). Draw an atomic view of the compound AB after it has undergone a physical change (such as evaporation). Draw an atomic view of the compound after it has undergone a chemical change (such as decomposition of AB into A and B).arrow_forwardThe explosion of an atomic bomb releases many radioactive isotopes, including strontium-90. Considering the location of strontium in the periodic table, suggest a reason for the fact that this isotope is particularly dangerous for human health.arrow_forwardWhat is the Process that produces elements in the stars? Give at least 10 sentecearrow_forward
- Identify the scientific law that is used in the process of balancing chemical equations. the law of conservation of mass the law of multiple proportions the law of constant composition Why should you not change the subscripts in chemical formulas when balancing chemical equations? Changing the subscripts in chemical formulas changes the physical state of the substance. Changing the subscripts in chemical formulas changes the amount of the substance. Changing the subscripts in chemical formulas changes the identity of the substance.arrow_forwardwhat is the physical state of element C? what is the physical appearance of element C? what is the color of element C?arrow_forwardIf green spheres represent chlorine atoms, yellow-green spheres represent fluorine atoms, white spheres represent hydrogen atoms, and all the molecules are gases, A.write the formula for each of the reactants. Express your answers as chemical formulas separated by a comma B. write the formula for each of the products. Express your answers as chemical formulas separated by a comma.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





