
Concept explainers
Interpretation: The illegal arrows in below set of arrows should be determined.
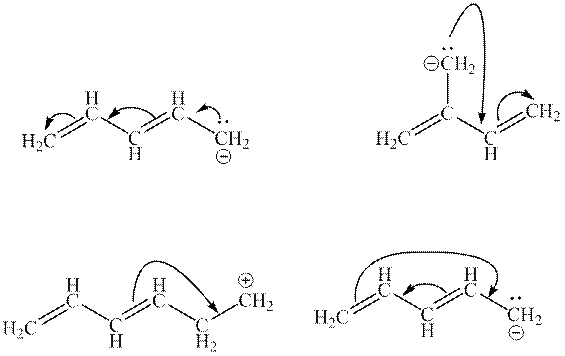
Concept introduction: Lewis structure is a representation of molecules in which dots are shown to represent unshared electrons and lines are shown to represent bonds. These lines and dots represent distribution of electrons in the molecule.
When one single structure is unable to describe all the properties of a single-molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for the resonance structure of anions in the movement of negative charge.
2. Use only arrow type 3 to move a positive charge for the resonance structure of cations.
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be the same in all resonance structures.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
- ! ( these are very easy please do all , if u hv plan to do only 3 plz skip, lets order do ) give typed ansarrow_forwardChemistry please label the function group present in the product ON THE PICTURE, according to the graph peaks.arrow_forwardGive typed full explanation of all three subpartsarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
