
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5, Problem 23CTQ
Interpretation Introduction
Interpretation: Whether
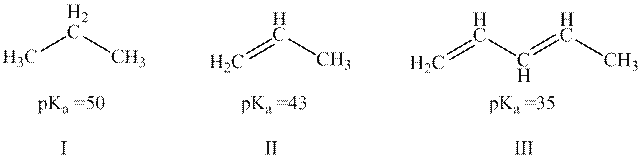
Concept introduction: The dissociation constant of acid represents strength of acid in solution. It is denoted by
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please help me find the answer to this reaction. Can you tell me step by step to find this answer. Thank you very much.
Which is the conjugate base in each of the pairs below?
Need help on this question.
is it that they are in the same category because of the strong OH activator ?
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 5 - Which elements on the periodic table (other than...Ch. 5 - You will not find “hydroxide” in the stockroom,...Ch. 5 - Prob. 3CTQCh. 5 - Prob. 4CTQCh. 5 - Prob. 5CTQCh. 5 - Prob. 6CTQCh. 5 - On which do you expect to have a more intense and...Ch. 5 - Prob. 8CTQCh. 5 - Prob. 9CTQCh. 5 - Prob. 10CTQ
Ch. 5 - Prob. 11CTQCh. 5 - Prob. 12CTQCh. 5 - Prob. 13CTQCh. 5 - Prob. 14CTQCh. 5 - Prob. 15CTQCh. 5 - Prob. 16CTQCh. 5 - For each proposed set of resonance structures: a....Ch. 5 - Consider the polarization of the C=O bond in the...Ch. 5 - The C=O double bond is called a “carbonyl bond.”...Ch. 5 - Prob. 20CTQCh. 5 - Prob. 21CTQCh. 5 - Prob. 22CTQCh. 5 - Prob. 23CTQCh. 5 - Prob. 24CTQCh. 5 - Prob. 25CTQCh. 5 - Prob. 26CTQCh. 5 - Prob. 27CTQCh. 5 - Prob. 28CTQCh. 5 - Prob. 29CTQCh. 5 - Prob. 30CTQCh. 5 - Prob. 31CTQCh. 5 - Confirm that there is no legitimate Lewis...Ch. 5 - Draw all resonance structures of the molecule...Ch. 5 - Prob. 34CTQCh. 5 - Prob. 35CTQCh. 5 - Prob. 36CTQCh. 5 - Occasionally, we will see an ionic compound that...Ch. 5 - Prob. 2ECh. 5 - Prob. 3ECh. 5 - Prob. 4ECh. 5 - Is it possible to draw a resonance structure of...Ch. 5 - Prob. 6ECh. 5 - Prob. 7ECh. 5 - Prob. 8ECh. 5 - Phenol (shown below) has a pKa10 . a. Based on pKa...Ch. 5 - Use curved arrows to show the most likely...Ch. 5 - Prob. 12ECh. 5 - Complete each Lewis structure, draw all important...Ch. 5 - Use curved arrows to show the most likely...Ch. 5 - Construct an explanation for why sulfuric acid is...Ch. 5 - Prob. 16ECh. 5 - Prob. 17ECh. 5 - Prob. 18E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the bases below would be best to ensure the following reaction was the major outcome?arrow_forwardThe following pKa values have been measured. Explain why a hydroxyl group in the para position decreases the acidity while a hydroxyl group in the meta position increases the acidity.arrow_forwardFor each molecule below, draw the conjugate acid or conjugate base or both if the molecule hasboth a conjugate acid and a conjugate base (e.g., water).arrow_forward
- Please answer this NEATLY, COMPLETELY, and CORRECTLY for an UPVOTE. Arrange the intermediates below in order of increasing basicity:arrow_forwarda) Draw the conjugate base of the compound shown. Include all resonance structures. b) The pKa of phenol is 10. Does the compound shown have a pKa greater or less than 10? Fully explain your answer. (Hint: “It’s more acidic” or “it’s less acidic” is not a full explanation)arrow_forwardPlease complete the following question. There is no need to provide an explaination. 6arrow_forward
- The SN2 reaction shows a reversible mechanism when the basic strength nucleophile and leaving group are similar, but not when the strength of the nucleophile as a base is greater than that of the leaving group. Explain.arrow_forward8 . Please answer this which is more acidicarrow_forwardPropose a synthesis for the following reaction Need answer step by steparrow_forward
- Rank the labeled protons (Ha~He) in order of increasing acidityarrow_forwardPredict the major product for the following as if the reaction was run at room temperature. The product is not a salt or acid.arrow_forwardHow would you carry out the following reaction? Please show the synthesis. Would like to confirm my answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
