
Concept explainers
Expand the list of orbitais considered in Figures 5.2 and 5.3 by using all three p orbitals of atom A and all five d orbitals of atom B. Which of these have the necessary match ofsymmetry for bonding and antibondingorbitals? These combinations are rarely seen in simplemolecules but can be important in transition metal complexes.
Interpretation: The list of orbitals should be expanded using all three p-orbitals of atom A and all five d-orbitals of atom B and the orbitals should be selected which has necessary match of symmetry for bonding and antibonding orbitals.
Concept Introduction: Three conditions are considered for overlapping which leads to bonding.
- The orbital’s symmetry must be such that regions with the same sign of ψ overlap.
- The energies of atomic orbital must be comparable.
- In order to provide good overlap, the distance between the atoms should be short enough but not so short as the repulsion of other electrons or nuclei occur.
Answer to Problem 5.1P
Bonding interactions -
P orbitals and
Explanation of Solution
The shapes of p orbitals are as follows:
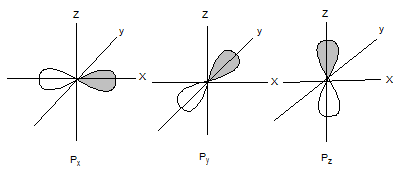
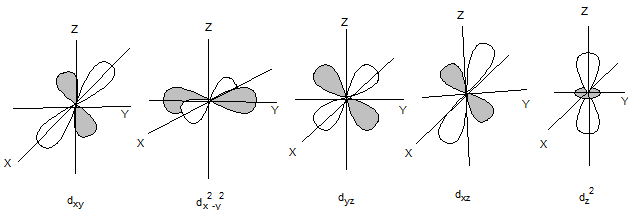
The shapes of d orbitals are as follows:
There are three possible bonding interactions. They are,
P orbitals and
Want to see more full solutions like this?
Chapter 5 Solutions
Inorganic Chemistry
Additional Science Textbook Solutions
General Chemistry: Atoms First
Chemistry (7th Edition)
Organic Chemistry
Organic Chemistry (9th Edition)
Chemistry For Changing Times (14th Edition)
- What are the point groups of a tetragonally compressed tetrahedral and square planar geometry of this complex?arrow_forwardUse resonance forms to show that the dipolar sigma complex shown in the sulfonationof benzene has its positive charge delocalized over three carbon atoms and its negativecharge delocalized over three oxygen atoms.arrow_forwardCFT is applicable to molecules in geometries other than octahedral. In octahedral complexes, remember that the lobes of the eg set point directly at the ligands. For tetrahedral complexes, the d orbitals remain in place,but now we have only four ligands located between the axes (as given). None of the orbitals points directly at the tetrahedral ligands. However, the eg set (along the Cartesian axes) overlaps with the ligands less than does the t2g set. By analogy with the octahedral case, predict the energy diagram for the d orbitals in a tetrahedral crystal field. To avoid confusion, the octahedral eg set becomes a tetrahedral e set, and the octahedral t2g set becomes a t2 set.arrow_forward
- Fine structure and hyperfine structure of atom with some example , in detail?arrow_forwardConstruct an MO correlation diagram for the complex ion[Ni(NH3)6]2+ and populate the orbitals with electrons. Do youexpect p bonding to be important for this complex? Whatfeatures of this diagram correspond to those predicted usingcrystal field theory? What kind of bonding would VB theorypredict for d8 complexes, and how does MO theory accountfor the bonding more accurately, even qualitatively?arrow_forwardCarbon monoxide is a good ligand and is toxic. Why is the isoelectronic N2molecule not toxic?arrow_forward
- One of the more striking hydride complexes is [ReH9]2-, which has tricapped trigonal prismatic geometry. Construct a representation using the hydrogen orbitals as a basis. Reduce this to its component irreducible representations, and indicate which orbitals of Re are of suitable symmetry to interact with the hydrogen orbitals.arrow_forward(a)Complex forming agents can be used to determine hardness of water how? Explain withthe help of following points. (i) Principle (ii) Chemical reactions (iii) Structure (iv)Calculations. Q.8(a)Write the MO electronic configuration of diatomic molecule having a bond order ofthree. If the electron pair following a bond between 2 atoms A and B is not in the center.Then what kind of bond is formed?(b) Although the outermost electronic configuration of transition elements is ns1or ns2likealkali and alkaline earth metal still they are not reactive like group 1&2 elements whyit is so?arrow_forwardWork out the stoichiometries and structures of the neutral complexes, chromium carbonyl, rhenium carbonyl, and nickel carbonyl using the 18-electron rule. Then use valence bond theory to determine the hybridizations of the orbitals in each complex?arrow_forward
- Why does the nitrosyl (NO+) ligand have a higher NO stretching frequency in the linear M-N-O geometry than in the bent M-N-O geometry? Group of answer choices In the linear geometry, N and O atoms have smaller masses than in the bent geometry In the linear geometry, there is an NO double bond while in the bent geometry, there is an NO triple bond In the linear geometry, N and O atoms have larger masses than in the bent geometry In the linear geometry, there is an NO triple bond while in the bent geometry, there is an NO double bondarrow_forwardDetermine the hybridization of the metal centre in the complexes below using Ligand Field Theory. Give explanations to the hybridization that happened.arrow_forwardIt is observed that the substitution reactions of square plane complexes generally follow the SN2 mechanism. Right?False?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





