
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 17P
Initial rate data for an enzyme that obeys Michaelis-Menten kinetics are shown in the following table. When the enzyme concentration is 3 nmol ml-1, a Lineweaver-Burk plot of this data gives a line with a y-intercept of 0.00426 (µmol- ml s).
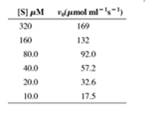
- Calculate kcat for the reaction.
- Calculate KM for the enzyme.
- When the reactions in part (b) are repeated in the presence of 12 µM of an uncompetitive inhibitor, the y-intercept of the Lineweaver-Burk plot is 0.352 (µmol-1 ml s). Calculate K’I for the inhibitor.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The turnover number for an enzyme that approximates Michaelis-Menten kinetics is known to be 500 min^-1. From the results shown in the table, enumerate Km and total amount of enzyme present. What is the Km for this enzyme? What is the Vmax for this enzyme? And what is the [E]T for this enzyme?
From a kinetics experiment, the Vmax was determined to be 450µM∙min-1. For the kinetic assay, 0.1mL of a 0.05mg/mL solution of enzyme was used, and the enzyme has a molecular weight of 125,000 g/mole. Assume a reaction volume of 700µL. Calculate the kcat (in sec-1) for the enzyme.
You obtain a calculated Vmax of 4.26uM/s and a Km of 122.5uM from a kinetics experiment performed using 0.5uM enzyme. What is the catalytic efficiency of this enzyme?
Chapter 8 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 8 - Prob. 1PCh. 8 - The enzyme urease catalyzes the hydrolysis of urea...Ch. 8 - An enzyme contains an active site aspartic acid...Ch. 8 - The folding and unfolding rate constants for a...Ch. 8 - In some reactions, in which a protein molecule is...Ch. 8 - Would you expect an “enzyme” designed to bind to...Ch. 8 - The initial rate for an enzyme-catalyzed reaction...Ch. 8 - a. If the total enzyme concentration in Problem 7...Ch. 8 - Prob. 9PCh. 8 - Prob. 10P
Ch. 8 - The following data describe the catalysis of...Ch. 8 - At 37 oC, the serine protease subtilisin has kcat...Ch. 8 - The accompanying figure shows three...Ch. 8 - The steady-state kinetics of an enzyme are studied...Ch. 8 - The same enzyme as in Problem 14 is studied in the...Ch. 8 - Enalapril is an anti-hypertension “pro-drug"...Ch. 8 - Initial rate data for an enzyme that obeys...Ch. 8 - Prob. 18PCh. 8 - Suggest the effects of each of the following...Ch. 8 - The inhibitory effect of an uncompetitive...Ch. 8 - Prob. 21PCh. 8 - Prob. 22PCh. 8 - Prob. 23PCh. 8 - In kinetics experiments, the hydrolysis of the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Lineweaver-Burk plots of enzyme kinetics for the reaction, S <-> P, has the following features: 1/v is zero when 1/[S] equals -40 liter mole^-1; 1/[S] is zero when 1/v equals 2.0 x 10^5 min mole^-1. What are the Vmax and Km? Vmax = 5 umol min^-1, Km = 2.5 mM? Vmax = 5 mmol min^-1, Km = 25 M? Vmax = 5 umol min^-1, Km = 25 mM? Vmax = 5 mol min^-1, Km = 2.5 mM? Vmax = 5 mol min^-1, Km = 25 mM?arrow_forwardFor a Michaelis-Menten enzyme, k1 = 5.2 ⅹ 108 M-1 s -1 , k-1 = 3.1 ⅹ 104 s -1 , and k2 = 3.4 ⅹ 105 s -1 . a) Write out the reaction, showing k1, k-1, and k2. Calculate Ks and Km. Does substrate binding approach rapid equilibrium or the steady state? Show work justify b) What is kcat for this reaction? Show work justify c) Calculate Vmax for the enzyme. The total enzyme concentration is 25 pmol L-1 , and each enzyme has two active sites.arrow_forwardAn enzyme that follows Michaelis-Menten kinetics has a KM value of 10.0 μM and a kcat value of 206 s−1 . At an initial enzyme concentration of 0.0100 μM , the initial reaction velocity was found to be 1.07×10−6 μM/s . What was the initial concentration of the substrate, [S] , used in the reaction ? Express your answer in micromolar to three significant figures..arrow_forward
- Lineweaver-Burk plots of enzyme kinetics for the reaction, S <-> P, has the following features: 1/v is zero when 1/[S] equals -40 liter mole^-1; 1/[S] is zero when 1/v equals 2.0 x 10^5 min mole^-1. What are the Vmax and Km?arrow_forwardAn enzyme catalyzes a reaction at a velocity of 20 μmol/min when the concentration of substrate (S)is 0.01 M. The Km for this substrate is 1 × 10-5 M. Assuming that Michaelis-Menten kinetics arefollowed, what will the reaction velocity be when the concentration of S is 1 ×10-6 M?arrow_forwardAn enzyme that follows simple Michaelis–Menten kinetics has an initial reaction velocity of 10 µmol⋅min-1 when the substrate concentration is five times greater than the KM. What is the Vmax of this enzyme in µmol⋅min−1?arrow_forward
- Assume that the experiments performed in the absence of inhibitors were conducted by adding 5 μL of a 2 mg/mL enzyme stock solution to an assay mixture with a total volume of 1 mL. Take into account that XYZase is a monomeric enzyme with a molecular mass of 45,000 Daltons. Hint: To calculate the ???? in units of per second (s−1), you must first determine the ???? in micromoles per second (μmol/sec). Please explain step by steparrow_forwardAn enzyme catalysed reaction has a Km of 8 mM and a Vmax of 13 nM.s-1. Use the Michaelis-Menten equation to calculate the reaction velocity when the substrate concentration is 18 mM.arrow_forwardQ is an analog of substrate A that binds to enzyme X and produces the following kinetics:[A] V0 (µmole/ml/min) [Q] = 0 [Q] = 0.5 µM [Q] = 2 µM1 µM 10 7 43 µM 20 16 1010 µM 35 31 2330 µM 43 41 3680 µM 47 46 43a) Plot the data in Lineweaver Burk plot form (Hint: there should be three plots of dataon your graph) and determine the following: the KM and Vmax of the enzyme X in theabsence of Q, and the KMapp the Vmaxapp at each concentration of Q. b) What type of inhibition is this?c) Calculate the inhibition constant (Ki) for Compound Qarrow_forward
- If you want to determine the KM for lactate, what protocol do you set up? Discuss the significance of the following kinetic parameters that are used to characterize enzyme activity: KM, Vmax, kcat, and kcat / KM.arrow_forwardFrom a kinetics experiment, kcat was determined to be 295sec-1. For the kinetic assay, 0.3mL of a 0.25mg/mL solution of enzyme was used, and the enzyme has a molecular weight of 125,000 g/mole. Assume a reaction volume of 3mL. Calculate Vmax (µM∙min-1) for the enzyme and catalytic efficiency ( in M-1sec-1) for the enzyme. The Km for the enzyme was determined to be 2.55 x 10-2M.arrow_forwardFrom a kinetics experiment, Kcat was determined to be 55sec^-1. For the kinetic assay, 0.05mL of a 0.05mg/mL solution of enzyme was used, and the enzyme has a molecular weight of 30,000g/mole. Assume a reaction volume of 3mL. Calculate Vmax (um*min^-1) for the enzyme and catalytic efficiency (in M^-1sec^-1) for the enzyme. The Km for the enzyme was determined to be 8.3*10^-2M.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Enzyme Kinetics; Author: MIT OpenCourseWare;https://www.youtube.com/watch?v=FXWZr3mscUo;License: Standard Youtube License