
Biochemistry: The Molecular Basis of Life
6th Edition
ISBN: 9780190209896
Author: Trudy McKee, James R. McKee
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 9, Problem 2Q
Summary Introduction
To review:
Identification of the redox reactions along with their reducing and oxidizing agents from the following reactions:
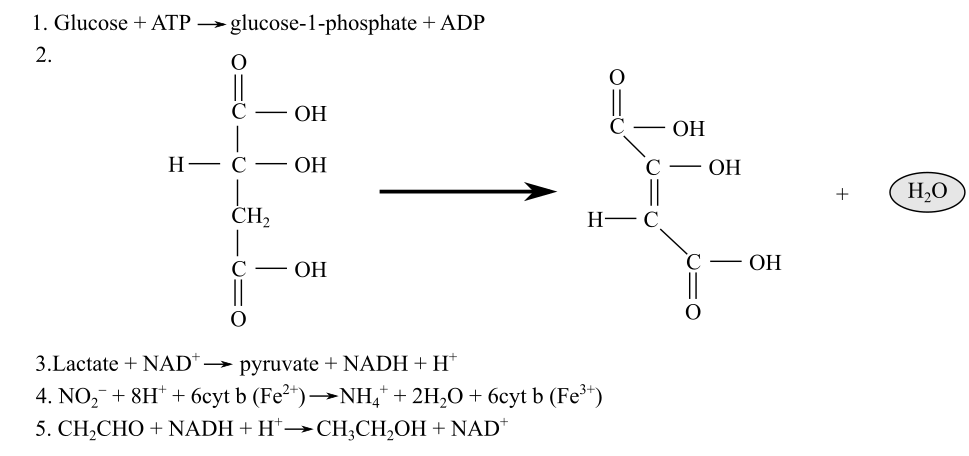
Introduction:
In living organisms, variousredox(
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Shown below are reduction potentials for four half-reactions. Which of the coupled reactions is favorable? (Note that for Cytochrome c you must multiply the reduction potential by 2 for each coupled reaction because only one electron is involved)
a) 2 Cytochrome c (Fe3+) + H2O ó 2 Cytochrome c (Fe2+) + O2
b) NADH + Succinate- ó NAD+ + Fumarate-
c) Fumarate- + H2O ó Succinate- + O2
d) All of the above
Under standard conditions, will the following reaction proceed spontaneously as written? Fumarate + NADH + H+⇌ succinate + NAD+
In the reaction catalyzed by malate dehydrogenase, which of the following molecules is classified as the reduced donor?
a. Malate
b. Oxaloacetate
c. Fumerate
d. FAD
e. FADH2
f. NAD+
g. NADH
Chapter 9 Solutions
Biochemistry: The Molecular Basis of Life
Ch. 9 - Prob. 1QCh. 9 - Prob. 2QCh. 9 - Prob. 3QCh. 9 - Prob. 4QCh. 9 - Prob. 5QCh. 9 - Prob. 6QCh. 9 - Prob. 7QCh. 9 - Prob. 1RQCh. 9 - Prob. 2RQCh. 9 - Prob. 3RQ
Ch. 9 - Prob. 4RQCh. 9 - Prob. 5RQCh. 9 - Prob. 6RQCh. 9 - Prob. 7RQCh. 9 - Prob. 8RQCh. 9 - Prob. 9RQCh. 9 - Prob. 10RQCh. 9 - Prob. 11RQCh. 9 - Prob. 12RQCh. 9 - Prob. 13RQCh. 9 - Prob. 14RQCh. 9 - Prob. 15RQCh. 9 - Prob. 16RQCh. 9 - Prob. 17RQCh. 9 - Prob. 18RQCh. 9 - Prob. 19RQCh. 9 - Prob. 20RQCh. 9 - Prob. 21RQCh. 9 - Prob. 22RQCh. 9 - Prob. 23RQCh. 9 - Prob. 24RQCh. 9 - Prob. 25RQCh. 9 - Prob. 26RQCh. 9 - Prob. 27RQCh. 9 - Prob. 28FBCh. 9 - Prob. 29FBCh. 9 - Prob. 30FBCh. 9 - Prob. 31FBCh. 9 - Prob. 32FBCh. 9 - Prob. 33FBCh. 9 - Prob. 34FBCh. 9 - Prob. 35FBCh. 9 - Prob. 36FBCh. 9 - Prob. 37FBCh. 9 - Prob. 38SACh. 9 - Prob. 39SACh. 9 - Prob. 40SACh. 9 - Prob. 41SACh. 9 - Prob. 42SACh. 9 - Prob. 43TQCh. 9 - Prob. 44TQCh. 9 - Prob. 45TQCh. 9 - Prob. 46TQCh. 9 - Prob. 47TQCh. 9 - Prob. 48TQCh. 9 - Prob. 49TQCh. 9 - Prob. 50TQCh. 9 - Prob. 51TQCh. 9 - Prob. 52TQCh. 9 - Prob. 53TQCh. 9 - Prob. 54TQCh. 9 - Prob. 55TQCh. 9 - Prob. 56TQCh. 9 - Prob. 57TQCh. 9 - Prob. 58TQCh. 9 - Prob. 59TQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following is NOT produced during the oxidative phase of the pentose phosphate shunt? a Ribulose-5-Phosphate b Glyceraldehyde-3-phosphate c CO2 d NADPHarrow_forwardComplete the balanced equation for the overall reaction by selecting an answer choice in the brackets. Sucrose + [2 Pi, 4Pi]+[4 ADP, 2 ADP, 4 ATP, 2ATP]+[2 NAD+, 4 NAD+, 6 NAD+]+[H2O, 5 H2O, 3 H2O] --> [2 cirate, 2 oxaloacetate, 2 pyruvate, 2 acetyl-coA]+[4 ADP, 2 ADP, 4 ATP, 2ATP] + [2 NAD+, 4 NAD+, 6 NAD+] + [2H+, 8H+, 6 H+, 4 H+, 10 H+] Does the commercial process require aerated culture medium—that is, is this a fermentation or an aerobic process? A. a fermentation process, because A. niger cells must use O2O2 to continuously regenerate NAD+ B. an aerobic process, because A. niger cells must use O2O2 to continuously regenerate NAD+ C. a fermentation process, because A. niger cells cannot use O2O2 to continuously regenerate NAD+ D. an aerobic process, because A. niger cells cannot use O2O2 to continuously regenerate NAD+arrow_forwardWhich of the following reactions is reversible and irreversible? Why? a. Pyruvate + β-hydroxybutyrate → lactate + acetoacetate b. Malate + pyruvate → oxaloacetate + lactate c. Acetaldehyde + succinate → ethanol + fumarate d. Pyruvate + NADH + H+ → lactate NAD+ e. Acetoacetate + NADH + H+ → β-hydroxybutyrate + NAD+ f. Malate + NAD+ → oxaloacetate + NADH + H+arrow_forward
- Using the glycerol-3-phosphate shuttle, determine how many ATP can be produced from one mole of each of the following compounds on complete oxidation?a.) Mannoseb.) Dihydroxyacetone phosphatec.) Citrated.) Malatee.) Succinatearrow_forwardA(n)___________ reaction converts glycerol to dihydroxyacetone. This reaction requires _________ and also produces __________. Blank 1 options- Oxidation-reduction Hydrolysis Isomerization Group transfer Internal rearrangement Blank 2 options- NAD+ and a dehydrogenase NADH and a dehydrogenase NAD+ and a phosphatase NADH and a phosphatase Blank 3 options- NADH and H+ NAD+ and H+ NADH and H2O NAD+ and H2Oarrow_forwardWhich of the following statements is true for the shown reaction? The reaction can occur in both cytosol and mitochondria Under starved conditions, the reaction becomes reversible to allow the synthesis of ketonebodies The reaction requires FMN as a cofactor Increase in NADH stimulates the reaction None of the abovearrow_forward
- Which of the following statements inaccurately describes glutamate dehydrogenase? Glutamate dehydrogenase uses either NAD+ or NADP+ in a redox reaction Glutamate dehydrogenase catalyzes an oxidative deamination reaction Glutamate dehydrogenase equilibrium lies with the reductive amination of glutamate Glutamate dehydrogenase utilizes hydrolysis to release ammonium from an imine intermediatearrow_forwardOzone in the lower atmosphere is a pollutant that can form by the following reaction involving the oxidation of unburned hydrocarbons:CH4(g) + 8 O2(g)-------->CO2(g) + 2 H2O(g) + 4 O3(g)Use the standard free energies of formation to determine ΔG°rxn for this reaction at 25 °C.arrow_forwardDefine the following terms: a. electron transport chain b. oxidation-reduction reactions c. conjugate redox pair d. reduction potential e. standard reduction potentialarrow_forward
- Which of the following statements is/are TRUE for the Krebs' cycle? Reaction 1: condensation of acetyl-CoA and oxaloacetate; produces H20. Reaction 3: oxidation of isocitrate to a-ketoglutarate; produces NADH and CO2. Reaction 6: oxidation of succinate to fumarate; produces FADH2 and CO2 Reaction 5: hydrolysis of succinyl-CoA to succinate; produces ATP.arrow_forwardWhich of the following enzymes consumes one molecule of NADH, during anaerobic fermentation in yeast, as it converts a two-carbon substrate molecule into a two-carbon product molecule? succinate dehydrogenase pyruvate decarboxylase C. isocitrate dehydrogenase lactate dehydrogenase alcohol dehydrogenasearrow_forwardwhich of the following glycolytic reactions is an oxidation? a) G3P to 1,3-BPG b) PEP to Pyr c) lactic acid fermentation under anaerobic conditions d) condensation of acetate with OAA e) succinate decarboxylase reactionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Anaerobic Respiration; Author: Bozeman Science;https://www.youtube.com/watch?v=cDC29iBxb3w;License: Standard YouTube License, CC-BY