
Concept explainers
The compound whose structure is shown here is acetyl acetone. It exists in two forms: the enol form and the keto form.
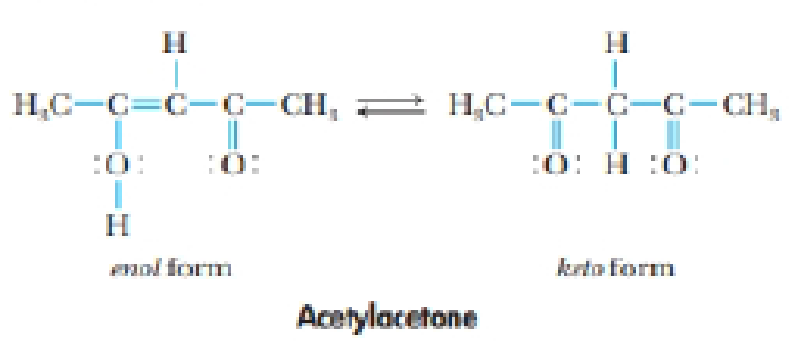
The molecule reacts with OH– to form an anion, [CH3COCHCOCH3] (often abbreviated acac– for acetylacetonate ion). One or the most interesting aspects of this anion is that one or more of them can react with transition metal cations to give stable, highly colored compounds.

(a) Are the keto and enol forms of acetylacetone resonance forms? Explain your answer.
(b) What is the hybridization or each atom (except H) in the enol form? What changes in hybridization occur when it is transformed into the keto form?
(c) What are the electron-pair geometry and molecular geometry around each C atom in the keto and enol forms? What changes in geometry occur when the keto form changes to the enol form?
(d) Draw three possible resonance structures for the acac– ion.
(e) Is cis-trans isomerism possible in either the enol or the keto form of acetylacetone?
(f) Is the enol form of acetylacetone polar? Where do the positive and negative charges lie in the molecule?
Trending nowThis is a popular solution!

Chapter 9 Solutions
Chemistry & Chemical Reactivity
- Does CH3COCH3 have any isomers? If so, show Lewis structures of them as well.arrow_forwardExplain how many chain isomers are possible for C4H10. Define their structure.arrow_forwardWhat exactly is the difference between (E)/(Z) and cis/trans nomenclature? In my class, we usually use (E)/(Z) for double bonds- is it possible to use it for single bonds? Is (E)/(Z) used when cis/trans is not able to depict the geometric bonding as there are four different groups?arrow_forward
- 1. A class organic compounds containing a six-membered ring with alternating double bonds that results in delocalization of the electrons within the ring. This class of compounds can have additional groups attached to the ring. 2. A family of organic compounds that can be represented by the general formula R-CHO. 3. A compound with the general formula R-COOH. It is a weak electrolyte that can lose an H+ ion in water solutions. 4. A hydrocarbon with a general formula of CnH(2n+2). All carbon-to-carbon bonds would be single bonds.arrow_forwardConsider cyclooctatetraene,C8H8, which has the octagonal structure shown belowWhat experiments or calculations could you perform to determinewhether cyclooctatetraene exhibits resonance?arrow_forwardWhat is the resonance form that describes the distribution of electrons in SeO2 ?arrow_forward
- What are 2 different molecules with the molecular formula (C7H14O2)arrow_forwardHow many valence electrons are there in a correctly drawn Lewis structure for formamide, HCONH2 ( You should also be able to draw the lewis structure for this. Hint, the C is in the center with an O ,N and 1 H attached. The other H atoms are attached to the N)arrow_forwardDraw a lewis structure for the molecular formula SiBr3- . What is the electronic geometry and molecular geometry around the Si atom in this molecule?arrow_forward
- How many isomers of all kinds the [Co(NH3)2(H2O)2(OH)2]+ has?arrow_forwardWhich compound of the following pairs would you expect to have the greatest dipole moment and by what? Draw its electrostatic potential map (without colors), indicating the direction of polarization of each link, using the convention δ + / δ- a) LiH or KH b) CH3NH2 or SiBr4arrow_forwardHow would you describe the molecular geometry of C2H2F2, and what are its bond angles?arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning



