
Organic And Biological Chemistry
7th Edition
ISBN: 9781305081079
Author: STOKER, H. Stephen (howard Stephen)
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.100EP
How many secondary carbon atoms are present in each of the structures in Problem 12-96?
a. 
b. 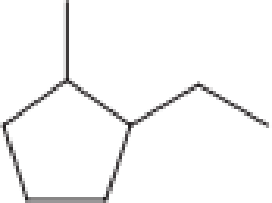
c. 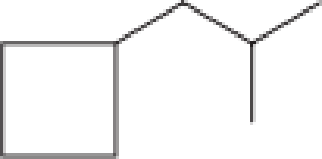
d. 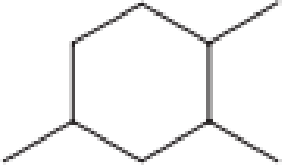
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is not correct about alkanes
a. They have relatively high boiling points
b. They have relatively low boiling points
c. They are hydrocarbons
d. They contain a lot of energy
Which of the following statement is true about alkanes?
a
Alkanes are one of the three unsaturated hydrocarbons.
b
Alkanes are always linear molecules.
c
Alkanes are commonly derived from petroleum and natural gas.
d
Alkanes have 4 additional hydrogens compared to an alkene with the same number of carbon.
Which of the following statements about alkanes, alkenes and alkynes is FALSE?A. Alkanes can be solids, liquids or gases.B. Alkenes are always gases in state at room temperature.C. Alkanes are more reactive to halogenation compared to alkenes.D. Alkynes exhibit the greatest electron density among hydrocarbons
Chapter 1 Solutions
Organic And Biological Chemistry
Ch. 1.1 - Prob. 1QQCh. 1.1 - Prob. 2QQCh. 1.2 - Which of the following statements about the...Ch. 1.2 - Prob. 2QQCh. 1.3 - Prob. 1QQCh. 1.3 - Prob. 2QQCh. 1.4 - Prob. 1QQCh. 1.4 - Prob. 2QQCh. 1.4 - Prob. 3QQCh. 1.5 - Prob. 1QQ
Ch. 1.5 - Prob. 2QQCh. 1.5 - Prob. 3QQCh. 1.6 - Prob. 1QQCh. 1.6 - Prob. 2QQCh. 1.6 - Prob. 3QQCh. 1.6 - Prob. 4QQCh. 1.7 - Prob. 1QQCh. 1.7 - Prob. 2QQCh. 1.8 - Prob. 1QQCh. 1.8 - Prob. 2QQCh. 1.8 - Prob. 3QQCh. 1.8 - Prob. 4QQCh. 1.8 - Prob. 5QQCh. 1.8 - Prob. 6QQCh. 1.8 - Prob. 7QQCh. 1.9 - Prob. 1QQCh. 1.9 - Prob. 2QQCh. 1.10 - Prob. 1QQCh. 1.10 - Prob. 2QQCh. 1.11 - Prob. 1QQCh. 1.11 - Prob. 2QQCh. 1.11 - Prob. 3QQCh. 1.12 - Prob. 1QQCh. 1.12 - Prob. 2QQCh. 1.12 - Prob. 3QQCh. 1.13 - Prob. 1QQCh. 1.13 - Prob. 2QQCh. 1.13 - Prob. 3QQCh. 1.14 - Prob. 1QQCh. 1.14 - Prob. 2QQCh. 1.14 - Prob. 3QQCh. 1.15 - Prob. 1QQCh. 1.15 - Prob. 2QQCh. 1.16 - Prob. 1QQCh. 1.16 - Prob. 2QQCh. 1.16 - Prob. 3QQCh. 1.17 - Prob. 1QQCh. 1.17 - Prob. 2QQCh. 1.17 - Prob. 3QQCh. 1.17 - Prob. 4QQCh. 1.18 - Prob. 1QQCh. 1.18 - Prob. 2QQCh. 1.18 - Prob. 3QQCh. 1.18 - Prob. 4QQCh. 1 - Prob. 1.1EPCh. 1 - Prob. 1.2EPCh. 1 - Prob. 1.3EPCh. 1 - Prob. 1.4EPCh. 1 - Indicate whether each of the following situations...Ch. 1 - Indicate whether each of the following situations...Ch. 1 - Prob. 1.7EPCh. 1 - Prob. 1.8EPCh. 1 - What is the difference between a saturated...Ch. 1 - What structural feature is present in an...Ch. 1 - Prob. 1.11EPCh. 1 - Prob. 1.12EPCh. 1 - Prob. 1.13EPCh. 1 - Prob. 1.14EPCh. 1 - Prob. 1.15EPCh. 1 - Prob. 1.16EPCh. 1 - Convert the expanded structural formulas in...Ch. 1 - Prob. 1.18EPCh. 1 - Prob. 1.19EPCh. 1 - Prob. 1.20EPCh. 1 - Prob. 1.21EPCh. 1 - Prob. 1.22EPCh. 1 - Prob. 1.23EPCh. 1 - Prob. 1.24EPCh. 1 - Prob. 1.25EPCh. 1 - Prob. 1.26EPCh. 1 - Indicate whether each of the following would be...Ch. 1 - Indicate whether each of the following would be...Ch. 1 - Prob. 1.29EPCh. 1 - Explain why two different straight-chain alkanes...Ch. 1 - With the help of Table 12-1, indicate how many...Ch. 1 - Prob. 1.32EPCh. 1 - How many of the numerous eight-carbon alkane...Ch. 1 - How many of the numerous seven-carbon alkane...Ch. 1 - For each of the following pairs of structures,...Ch. 1 - For each of the following pairs of structures,...Ch. 1 - Convert each of the following linear condensed...Ch. 1 - Prob. 1.38EPCh. 1 - Prob. 1.39EPCh. 1 - Prob. 1.40EPCh. 1 - Prob. 1.41EPCh. 1 - Prob. 1.42EPCh. 1 - Prob. 1.43EPCh. 1 - Prob. 1.44EPCh. 1 - Prob. 1.45EPCh. 1 - Prob. 1.46EPCh. 1 - Prob. 1.47EPCh. 1 - Prob. 1.48EPCh. 1 - Prob. 1.49EPCh. 1 - Prob. 1.50EPCh. 1 - Prob. 1.51EPCh. 1 - Prob. 1.52EPCh. 1 - Draw a condensed structural formula for each of...Ch. 1 - Draw a condensed structural formula for each of...Ch. 1 - Prob. 1.55EPCh. 1 - For each of the alkanes in Problem 12-54,...Ch. 1 - Explain why the name given for each of the...Ch. 1 - Prob. 1.58EPCh. 1 - Indicate whether or not the two alkanes in each of...Ch. 1 - Prob. 1.60EPCh. 1 - How many of the 18 C8 alkane constitutional...Ch. 1 - How many of the nine C7 alkane constitutional...Ch. 1 - Prob. 1.63EPCh. 1 - Prob. 1.64EPCh. 1 - Prob. 1.65EPCh. 1 - Prob. 1.66EPCh. 1 - Do the line-angle structural formulas in each of...Ch. 1 - Do the line-angle structural formulas in each of...Ch. 1 - Convert each of the condensed structural formulas...Ch. 1 - Convert each of the condensed structural formulas...Ch. 1 - Assign an IUPAC name to each of the compounds in...Ch. 1 - Prob. 1.72EPCh. 1 - Prob. 1.73EPCh. 1 - Prob. 1.74EPCh. 1 - For each of the alkane structures in Problem...Ch. 1 - For each of the alkane structures in Problem...Ch. 1 - Prob. 1.77EPCh. 1 - Prob. 1.78EPCh. 1 - Prob. 1.79EPCh. 1 - Prob. 1.80EPCh. 1 - Prob. 1.81EPCh. 1 - Prob. 1.82EPCh. 1 - Draw condensed structural formulas for the...Ch. 1 - Draw condensed structural formulas for the...Ch. 1 - To which carbon atoms in a hexane molecule can...Ch. 1 - Prob. 1.86EPCh. 1 - Prob. 1.87EPCh. 1 - Prob. 1.88EPCh. 1 - Give an acceptable alternate name for each of the...Ch. 1 - Prob. 1.90EPCh. 1 - Prob. 1.91EPCh. 1 - Prob. 1.92EPCh. 1 - Prob. 1.93EPCh. 1 - Prob. 1.94EPCh. 1 - What is the molecular formula for each of the...Ch. 1 - Prob. 1.96EPCh. 1 - Prob. 1.97EPCh. 1 - Prob. 1.98EPCh. 1 - Prob. 1.99EPCh. 1 - How many secondary carbon atoms are present in...Ch. 1 - Assign an IUPAC name to each of the following...Ch. 1 - Assign an IUPAC name to each of the following...Ch. 1 - Prob. 1.103EPCh. 1 - Prob. 1.104EPCh. 1 - Prob. 1.105EPCh. 1 - Prob. 1.106EPCh. 1 - What is the molecular formula for each of the...Ch. 1 - Prob. 1.108EPCh. 1 - Prob. 1.109EPCh. 1 - Prob. 1.110EPCh. 1 - Prob. 1.111EPCh. 1 - Prob. 1.112EPCh. 1 - Determine whether cistrans isomerism is possible...Ch. 1 - Prob. 1.114EPCh. 1 - Prob. 1.115EPCh. 1 - Prob. 1.116EPCh. 1 - Prob. 1.117EPCh. 1 - Indicate whether the members of each of the...Ch. 1 - Prob. 1.119EPCh. 1 - Prob. 1.120EPCh. 1 - Prob. 1.121EPCh. 1 - Prob. 1.122EPCh. 1 - Prob. 1.123EPCh. 1 - Which member in each of the following pairs of...Ch. 1 - Prob. 1.125EPCh. 1 - Prob. 1.126EPCh. 1 - Answer the following questions about the...Ch. 1 - Prob. 1.128EPCh. 1 - Prob. 1.129EPCh. 1 - Prob. 1.130EPCh. 1 - Write molecular formulas for all the possible...Ch. 1 - Write molecular formulas for all the possible...Ch. 1 - Prob. 1.133EPCh. 1 - Prob. 1.134EPCh. 1 - Prob. 1.135EPCh. 1 - Assign an IUPAC name to each of the following...Ch. 1 - Prob. 1.137EPCh. 1 - Prob. 1.138EPCh. 1 - Prob. 1.139EPCh. 1 - Prob. 1.140EPCh. 1 - Prob. 1.141EPCh. 1 - Prob. 1.142EPCh. 1 - Prob. 1.143EPCh. 1 - Prob. 1.144EPCh. 1 - Prob. 1.145EPCh. 1 - Prob. 1.146EPCh. 1 - Give the IUPAC names for the eight isomeric...Ch. 1 - Prob. 1.148EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each of the alkane structures in Problem 12-51, give the number of (a) primary, (b) secondary, (c) tertiary, and (d) quaternary carbon atoms present. a. b. c. d.arrow_forwardAs stated in Section 11-9, the wax found in apple skins is an unbranched alkane with the molecular formula C^H^. Explain how the presence of this alkane in apple skins prevents the loss of moisture from within the apple.arrow_forwardWhy are different conformations of an alkane not considered structural isomers?arrow_forward
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License