
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.23SP
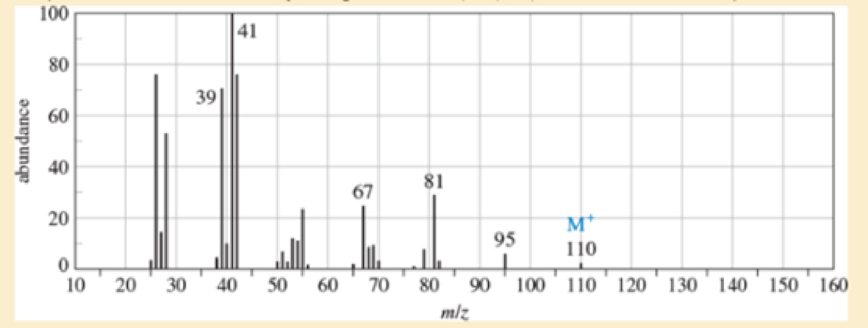
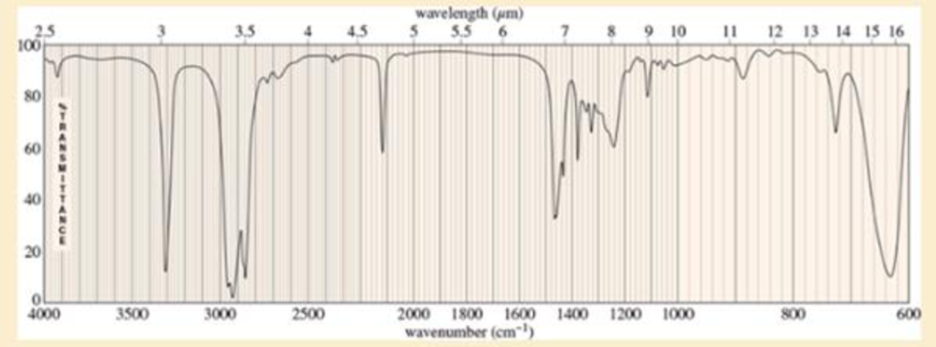
An unknown, foul-smelling hydrocarbon gives the mass spectrum and infrared spectrum shown.
- a. Use the mass spectrum to propose a molecular formula. How many elements of unsaturation are there?
- b. Use the IR spectrum to determine the functional group(s), if any.
- c. Propose one or more structures for this compound. What parts of the structure are uncertain? If you knew that hydrogenation of the compound gives n-octane, would the structure still be uncertain?
- d. Propose structures for the major fragments at 39, 67, 81, and 95 in the mass spectrum.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
How can you distinguish aldehydes, esters and carboxylic acids using IR spectra? Explain using specific examples.
From your results, which component had the highest Rf value and which had the lowest?
Based on your answer from the previous question, what could be the reason behind some components having an Rf value higher than the others? Compare the qualities of the two components that rendered the highest and lowest Rf
After performing the simulation, what do you think are the possible practical applications of chromatographic techniques that are relevant to your program NURSING?
what makes aromatic and aliphatic ethers different from each other in mass spectrometry
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Real walls are never totally adiabatic. Use your experience to order the following walls in increasing order wi...
Thermodynamics, Statistical Thermodynamics, & Kinetics
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
Problem 11.1 Neopheliosyne B is a novel acetylenic fatty acid isolated from a New Caledonian marine sponge. (a)...
Organic Chemistry
2. Why shouldn’t you work in a laboratory by yourself?
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The oxidation of 3-pentanol to 3-pentanone was performed in the lab and an IR was taken of the finished product. (a) what are the key functional groups in the starting material, 3-pentaol? (b) what are the key functional groups in the product, 3-pentanone? (c) based on the IR spectrum below, was the experiment successful, and, if so, how pure was the product? Explain your reasoning.arrow_forwardHow to get the wt. sample, g.arrow_forwardBased on this IR spectra what functional groups are present? The spectra was run for a sample of 2'-hydroxy-4-methoxychalconearrow_forward
- Identify the IR and analyze the mass spec and find the working structure and NMRarrow_forwardWhich compound gives rise to a major [M − 18] peak in the mass spectrum? Group of answer choices A. 1-bromoheptane B. 4-methylheptane C. 2-heptanol D. 3-ethylheptanearrow_forwardAnalyze the spectra and write the name of compound. Also write brief explanation for each spectra verifying the parts of your suggested molecule ( for IR indicate the functional groups which match is the peaks. for mass indicate the fragments. for CNMR an HNMR indicate which peak belongs to what).arrow_forward
- Which of the following functional group can be predicted with the IR spectrum shown? Question 22 options: Amine Alcohol Alkyne Alkene Ketonearrow_forwardOrganic Chemistry: IR spectroscopy A student obtains an IR spectrum with an intense, broad absorption at about 3350 cm-1. The student claims that the sample is an alcohol, but the professor says that the absorption is there because the sample is contaminated with water. What peak(s) could be looked for to show that the sample is indeed an alcohol? Which functional groups would make it difficult to use this strategy?arrow_forwardThe IR spectrum below reveals what functional group, if any? give explanation for your choice.arrow_forward
- Chemistry I need help with a homework problem. A particular organic molecule has a weight of 92 and provides the given mass spectrum below. a. Draw the structure of the molecule that matches the graph below. b. Demonstrate the electron-pushing arrows that account for the given fragmentation masses.arrow_forwardAccount for the formation of the base peaks in these mass spectra. Q.) Isobutylmethylamine, m/z 44arrow_forwardHow would you interpret this IR spectra of fluorene? What I mean by this is which peaks let you know its fluorene and which peaks show impurities if any such as 9-fluorenone since this lab required a separation of fluorene and 9-fluorenone. Other impurities could be solid alumina and sand and the solvents of pet. ether and dichlormethane.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY