
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.31SP
Consider the following four structures, followed by mass spectral data. Match each structure with its characteristic molecular ion or fragment. In each case, give a likely structure of the ion responsible for the base peak.
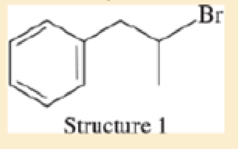
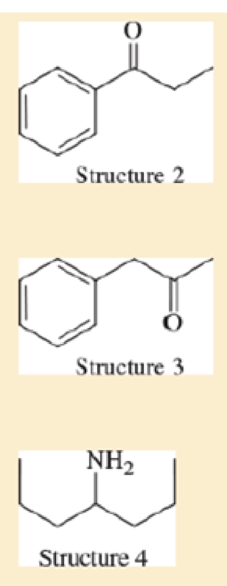
- a. base peak at 105
- b. base peak at 72
- c. M+ doublet at 198 and 200, base peak at 91
- d. base peak at 91, large peak at 43
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider isomeric alcohols A and B and mass spectra [1] and [2].(a) Label the molecular ion and base peak in each spectrum. (b) Use the fragmentation patterns to determine which mass spectrum corresponds to isomer A and which corresponds to isomer B.
Which of the following molecules matches the given data?
(Note: All the molecules have a M+ = m/z 102)
C5H10O2
base peak = m/z 43
Identify the important peaks in the following MS spectral data and draw the structure of the important peaks in the following MS spectral data.
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (14th Edition)
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
The smallest building blocks inside your cell phone are about 1000 times smaller than the diameter of a human h...
Chemistry In Context
Real walls are never totally adiabatic. Use your experience to order the following walls in increasing order wi...
Thermodynamics, Statistical Thermodynamics, & Kinetics
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each structure below, use numbers to indicate chemically equivalent and distinct hydrogens,and make a table showing the predicted integration and multiplicity of each peak cluster.arrow_forwardFollowing is the mass spectrum of an unknown compound. The two highest peaks are at m/z 120 and 122. Suggest a structure for this compound. (Data from http://webbook.nist.gov/chemistry/.)arrow_forwardHere are proton NMR data for 1-bromopropane: Ha : triplet (2H) 3.32ppm; Hb : multiplet (2H)1.81ppm; Hc : triplet (3H) 0.93ppm. (Relative integrations shown in parentheses.) a. Through how many bonds can a hydrogen split another hydrogen? b. According to this splitting rule, does Ha split Hc ? c. Is your answer in part a) consistent with the multiplicity listed for peak clusters a and c? d. How many hydrogenssplit Hb ? e. Upon very close inspection of the proton NMR spectrum of 1-bromopropane, you wouldfind that peak cluster b has at least six peaks. Is this consistent with your answer in part d)? f. Speculate as to why any peak cluster with more than four peaks is listed simply as a"multiplet."arrow_forward
- Calculate the IHD and identify the important peaks in the following MS spectral data and draw the structure of the important peaks in the following MS spectral data.arrow_forwardAn unknown hydrocarbon has a molecular ion peak at m/e = 84, with a relative intensity of 31.3. The M+1 peak has a relative intensity of 2.06, and the M+2 peak has a relative intensity of 0.08. What is the molecular formula of this substance?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY