
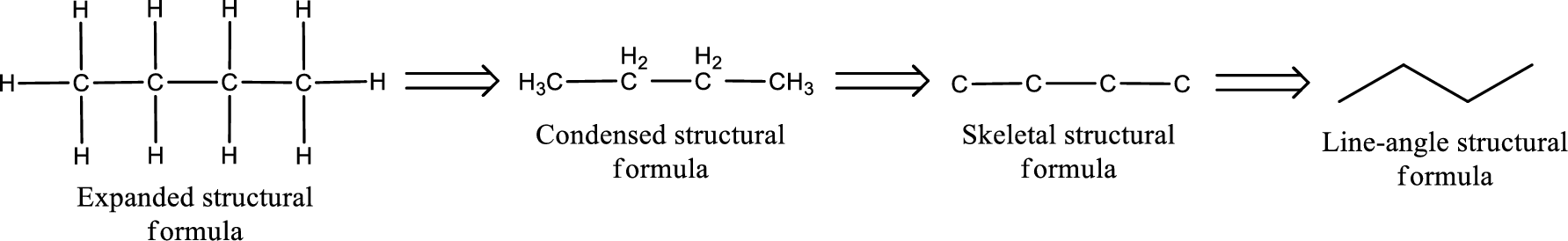
(a)
Interpretation:
Structural formula for the given
Concept Introduction:
Structure of the given aldehyde can be drawn from the IUPAC name. In the IUPAC name, the parent chain of carbon atom can be identified and then the substituents present in it can also be identified. With these information, the structure for the given compound can be drawn. In an aldehyde the counting has to be always from the carbonyl carbon that is given the number 1.
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
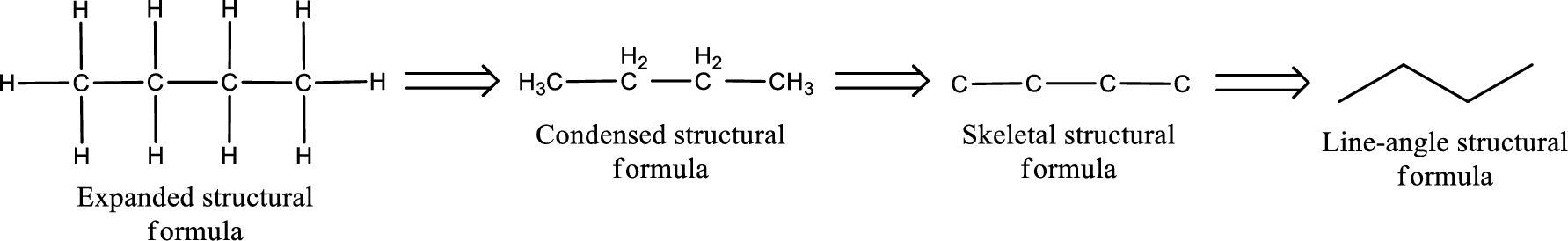
(b)
Interpretation:
Structural formula for the given aldehyde has to be drawn.
Concept Introduction:
Structure of the given aldehyde can be drawn from the IUPAC name. In the IUPAC name, the parent chain of carbon atom can be identified and then the substituents present in it can also be identified. With these information, the structure for the given compound can be drawn. In an aldehyde the counting has to be always from the carbonyl carbon that is given the number 1.
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
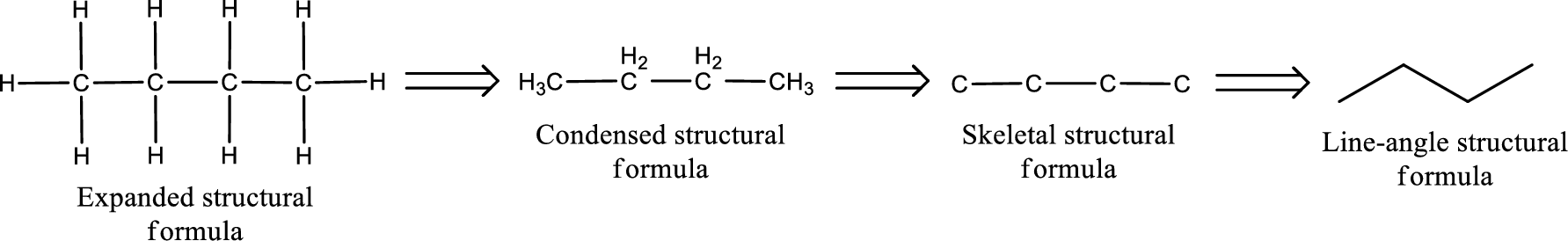
(c)
Interpretation:
Structural formula for the given aldehyde has to be drawn.
Concept Introduction:
Structure of the given aldehyde can be drawn from the IUPAC name. In the IUPAC name, the parent chain of carbon atom can be identified and then the substituents present in it can also be identified. With these information, the structure for the given compound can be drawn. In an aldehyde the counting has to be always from the carbonyl carbon that is given the number 1.
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
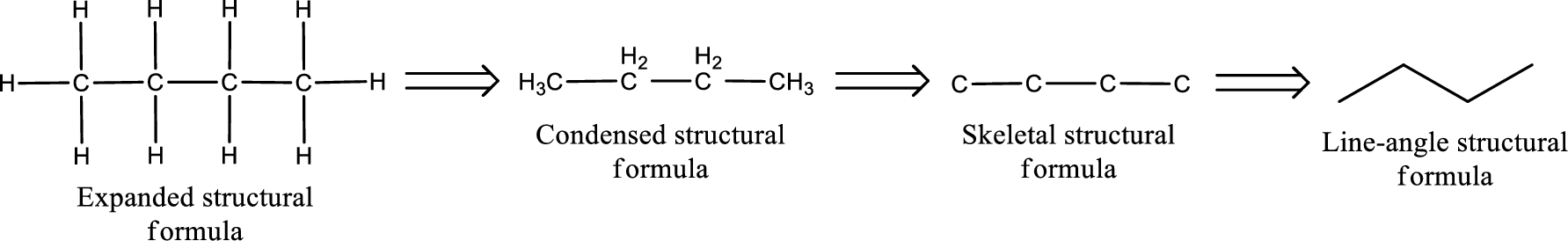
(d)
Interpretation:
Structural formula for the given aldehyde has to be drawn.
Concept Introduction:
Structure of the given aldehyde can be drawn from the IUPAC name. In the IUPAC name, the parent chain of carbon atom can be identified and then the substituents present in it can also be identified. With these information, the structure for the given compound can be drawn. In an aldehyde the counting has to be always from the carbonyl carbon that is given the number 1.
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
Trending nowThis is a popular solution!

Chapter 15 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- Propose a scheme to separate and isolate a mixture containing 4-aminobenzoate, 1,2,4,5-tetrachlorobenzene, and napthalene. Isolate two of the three solids by extraction. (please explain what solvents to use and the process step by step)arrow_forwardMestranol, sold under the brand names Enovid, Norinyl, and Ortho-Novum among others, is an estrogen medication which has been used in birth control pills. Select all of the functional group families, to which mestranol belongs. Choose one or more: O A. ester OB. alkene OC. ether O D. thiol O E. alcohol OF. arene OG. aldehyde OH. ketone O 1. alkyne OHarrow_forwardClassify the following lipid (choose all that apply for the overall structure, not the individual residues). a. wax ester b. fatty acid c. polyunsaturated d. trans alkene(s) e. steroid f. monounsaturated g. triglyceride h. cis alkene(s) i. saturatedarrow_forward
- Draw the structure of the wax composed of stearic acid and a straight-chained alcohol with 30 carbon atoms.arrow_forwardDraw the enantiomer structure of the following given carbohydrates. Be sure to include appropriate D or L specification.arrow_forwardDrawn are four isomeric dimethylcyclopropanes. How are the compounds in each pair related (enantiomers, diastereomers, constitutional isomers): A and B; A and C; B and C; C and D?arrow_forward
- Write the formulas of the four singly chlorinated isomers formed when 2-methylbutane reacts with Cl2 in the presence of light.arrow_forwardNinhydrin is a compound that is commonly used in forensic identification, because it turns purple in contact with the amino acids often found in sweat residue. Using the structure provided, answer the questions below. b. How many carboxylic acid functional groups are present? Explain your answer OH ΠOH 2arrow_forwardClassify the following lipid (choose all that apply).CHOOSE ALL THAT APPLY a. omega-6 b. trans alkene(s) c. omega-3 d. wax ester e. saturated f. monounsaturated g. fatty acid h. triglyceride i. steroid j. polyunsaturated k. cis alkene(s)arrow_forward
- Determine the chemical formula for the following molecules by counting the Carbon, Hydrogen and Oxygen atoms. Determine if the molecule is a carbohydrate by checking the ratio of atoms. 5. Ho C 1 H-C-OH H-C-OH H-C-OH CH₂OH 6. CH₂OH HO-C=0 [ H-C-OH H-C-OH 1 H-C-DH I H - COH I H-C-H I H Chemical formula Carbohydrate ? Chemical formula Carbohydrate ?arrow_forwardWhat functional groups are present in hydrothol (herbicide)?arrow_forwardNinhydrin is a compound that is commonly used in forensic identification, because it turns purple in contact with the amino acids often found in sweat residue. Using the structure provided, answer the questions below. b. How many carboxylic acid functional groups are present? Explain your answer. OH OHarrow_forward
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education





