
Concept explainers
(a)
Interpretation:
Acceptable name for the following compound should be determined:
CH3(CH2)8CO2CH3
Concept Introduction:
All three carboxylic acids, esters and amides contain carbonyl group (carbon atom connects to oxygen atom via double bond).
In carboxylic acids, there has a carboxylic group (COOH).
In esters, there has an alkoxy group (OR') bonded to the carbonyl carbon.
In amides, there has a nitrogen atom bonded to the carbonyl carbon.
(b)
Interpretation:
Acceptable name for the following compound should be determined:
(CH3)2CHCH2CONH2
Concept Introduction:
All three carboxylic acids, esters and amides contain carbonyl group (carbon atom connects to oxygen atom via double bond).
In carboxylic acids, there has a carboxylic group (COOH).
In esters, there has an alkoxy group (OR') bonded to the carbonyl carbon.
In amides, there has a nitrogen atom bonded to the carbonyl carbon.
(c)
Interpretation:
Acceptable name for the following compound should be determined:
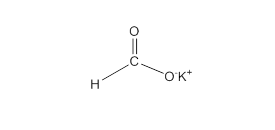
Concept Introduction:
All three carboxylic acids, esters and amides contain carbonyl group (carbon atom connects to oxygen atom via double bond).
In carboxylic acids, there has a carboxylic group (COOH).
In esters, there has an alkoxy group (OR') bonded to the carbonyl carbon.
In amides, there has a nitrogen atom bonded to the carbonyl carbon.
(d)
Interpretation:
Acceptable name for the following compound should be determined:
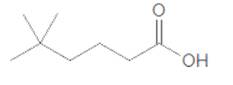
Concept Introduction:
All three carboxylic acids, esters and amides contain carbonyl group (carbon atom connects to oxygen atom via double bond).
In carboxylic acids, there has a carboxylic group (COOH).
In esters, there has an alkoxy group (OR') bonded to the carbonyl carbon.
In amides, there has a nitrogen atom bonded to the carbonyl carbon.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
General, Organic, and Biological Chemistry - 4th edition
- What mass of 2-bromopropane could be prepared from 25.5 g of propane? Assume a 100% yield of product.arrow_forward3. a. What is the chemical structure of benzoic acid, circle functional groups different than alkane,alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? __________________________arrow_forward1. What does the ff compound belong to: a. ketone b. alcohol c. aldehyde d. alkane 2. What group does the ff organic compound belong? a. ketone b. carboxylic acid c. aldehyde d. alkyl halide 3. What group does the ff organic compound belong? a. alkene b. ketone c. alcohol d. aldehydearrow_forward
- HCl (aq), Zn(Hg) Br2, FeBr3 NBS, light KMnO4, H3O+ Mg metal, ether KOH, EtOH, heat CH3Cl, AlCl3 Dilute H3O+ ClCO(CH2)2CH3, AlCl3 NaCCCH2CH3 HNO3, H2SO4 2-butanonearrow_forwardWhat is the name of the alkene? CH3CH=CHCH=CH2arrow_forwardPurification of Organic Compounds What type of organic compounds can be purified by distillation?arrow_forward
- Give iupac name for this diol CH3CH(OH)(CH2)4CH(OH)C(CH3)3arrow_forwardIs CH3F or CH3CH2OH more soluable in water.arrow_forward1. what group does the ff organic compound belong? a. organic nitrite b. esters c. amines d. nitriles 2. what group does the ff organic compound belong? a. alcohol b. ketone c. aldehyde d.alkene 3. what group does the ff organic compound belong? a. carboxylic acid b. alcohol c. aromatic d. ketonearrow_forward
- What kind(s) of intermolecular forces are present in alcohols?a.covalent b.ionic c.dispersion d.hydrogen bonding e.dipole-dipole. What kind(s) of intermolecular forces are present in alkanes?a.covalent b.ionic c.dispersion d.hydrogen bonding e.dipole-dipole.arrow_forwardA solution contains 10.00 g of a non–volatile hydrocarbon in 100.0 g of acetone. The boiling point of the pure acetone is 55.95 ⁰C; the boiling point of the solution is 59.00⁰C.The boiling point constant of acetone is 1.71 ⁰C kg/mol. i.What is the molar mass of the hydrocarbon?ii.What is the formula of the hydrocarbon?iii.Draw all of the isomers of the hydrocarbon.arrow_forwardDraw an isomer of C5H10O that contains an alcoholarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning  Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





