
(a)
Interpretation:
The acceptable name for the following amide should be determined:
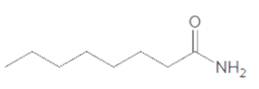
Concept Introduction:
An organic compound in which carboxy functional group that is -COOH is bonded to the carbon atom is said to be a carboxylic acid. The general formula for carboxylic acid is RCOOH or RCO2H. When -OH (hydroxyl group) of the carboxylic acid is replaced by nitrogen (-N) then it results in the formation of an amide.
The reaction which results in the formation of amide along with water on heating acids with
So, in order to give the IUPAC name to the amides, the rules for naming carboxylic acid is followed and -oic acid of the carboxylic acid is replaced by amide.
In order to give the name to the amide group, the following steps are followed:
- The parent (longest)
alkane chain is named as for carboxylic acids. - The -oic acid in the name is changed to -amide.
- The numbering of the chain is done in such a way that amide group and substituents gets the smaller number.
- N-alkyl is used to show each alkyl group bonded to -N atom in the name for secondary and tertiary amides.
For number of carbons atoms chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
In order to write the common name of the amides, the common of acids are written from which the amide has been formed by replacing -oic acid in name from -amide.
(b)
Interpretation:
The acceptable name for the following amide should be determined:
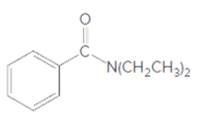
Concept Introduction:
An organic compound in which carboxy functional group that is -COOH is bonded to the carbon atom is said to be a carboxylic acid. The general formula for carboxylic acid is RCOOH or RCO2H. When -OH (hydroxyl group) of the carboxylic acid is replaced by nitrogen (-N) then it results in the formation of an amide.
The reaction which results in the formation of amide along with water on heating acids with amine or ammonia is said to be amidation.
So, in order to give the IUPAC name to the amides, the rules for naming carboxylic acid is followed and -oic acid of the carboxylic acid is replaced by amide.
In order to give the name to the amide group, the following steps are followed:
- The parent (longest) alkane chain is named as for carboxylic acids.
- The -oic acid in the name is changed to -amide.
- The numbering of the chain is done in such a way that amide group and substituents gets the smaller number.
- N-alkyl is used to show each alkyl group bonded to -N atom in the name for secondary and tertiary amides.
For number of carbons atoms chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
In order to write the common name of the amides, the common of acids are written from which the amide has been formed by replacing -oic acid in name from -amide.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Cocaine is a widely abused, addicting drug. Cocaine is usually obtainedas its hydrochloride salt (cocaine hydrochloride) but can be converted tocrack (the neutral organic molecule) by treatment with base. How does the relative solubility explain why crack is usuallysmoked but cocaine hydrochloride is injected directly into thebloodstream?arrow_forwardN-p-hydroxyphenylethanamide is commonly known as a. acetaminophen b. acetamide c. acetanilide d. formamide High molar mass amines have __________ odor. a.strong ammoniacal b.fruity c.fishy d.obnoxious Trimethyl amine has _________ odor. a.obnoxious b.fishy c. ammoniacal d. fruityarrow_forwardwhat Trade Name (Brand Name) of Arecoline ?arrow_forward
- Synthadotin is a promising anticancer drug in clinical trials. a.Identify the functional groups. b.Classify any amine or amide as 1°, 2°, or 3°. c.At which sites can synthadotin hydrogen bond to another molecule like itself? d. Label two nucleophilic sites. e. Label two electrophilic sites. f. What product is formed when synthadotin is treated with HCl?arrow_forwardShow how to convert hexanoic acid to given aminearrow_forwardPredict whether Tolualdehyde will test positive for a) Tollens’ test, b) Benedict’s test. Pls explain why.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

