
(a)
Interpretation:
The name of the following salt of carboxylic acid should be determined:
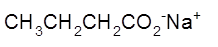
Concept Introduction:
The structural formula represents all the bonded atoms with
(b)
Interpretation:
The name of following salt of carboxylic acid should be determined:
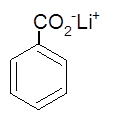
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds. The molecular formula of an organic compound represents the number of bonded atoms with their atomic symbols.
The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule. IUPAC purposed some rules to determine the name of an organic compound that is based on the number of C atoms in the longest chain of the compound and the name of branches.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Define the structure of a carboxylic acid ?arrow_forward1. what grouo does the ff organic compound belong? a. ketone b. ether c. cyloalkane d. esther 2. what group does the ff organic compound belong? a. amide b. azo c. nitrile d. amine 3. what is the priority functional group of the ff organic compound? a. carboxyl b. hydroxyl c. carbonyl d. hydroxidearrow_forward1. What does the ff compound belong to: a. ketone b. alcohol c. aldehyde d. alkane 2. What group does the ff organic compound belong? a. ketone b. carboxylic acid c. aldehyde d. alkyl halide 3. What group does the ff organic compound belong? a. alkene b. ketone c. alcohol d. aldehydearrow_forward
- What elements compose: a) the hydroxyl group b) the carbonyl group c) an aldehyde group d) a ketone grouparrow_forwardAn anhydride is an organic molecule derived from two carboxylic acids. What are these two acids for the molecule in the picture? i. Two butanoic acidii. One octanoic acidiii. Butanoic acid + propanoic acidiv. Acetic acid + butanoic acidarrow_forwardAcid Alcohol Odor Structure salicylic acid methanol wintergreen ? anthranilic acid methanol Grape ?arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




