
(a)
Interpretation:
The main product of the mononitration of benzoic acid should be predicted.
Concept introduction:
The electrophilic
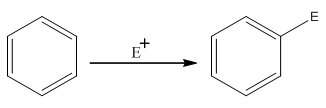
Where,
The activating groups are the groups that have the ability to donate the electron density to the benzene ring.
The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta direction groups are deactivating groups.
The nitration reaction takes place in the presence of nitric acid and sulphuric acid. In this reaction, the protonation of nitric acid occurs in order to produce the nitronium ion. The nitronium ion will attach on the benzene ring to form nitrobenzene. The general reaction is as follows:
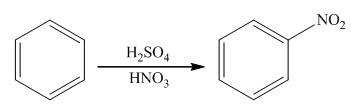
(b)
Interpretation:
The main product of the monosulphonation of phenol should be predicted.
Concept introduction:
The electrophilic aromatic substitution is the type of reaction in which an electrophile substitutes the hydrogen atom of benzene. A general electrophilic aromatic substitution reaction of benzene can be written as:
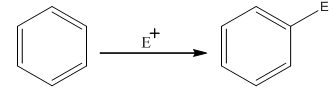
Where,
The activating groups are the groups that have the ability to donate the electron density to the benzene ring.
The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta direction groups are deactivating groups.
The sulfonation takes place in the presence of sulphuric acid. In this reaction, sulfur trioxide is formed that acts as an electrophile. Sulfur trioxide will attach on the benzene ring to form the final product. The general reaction is as follows:
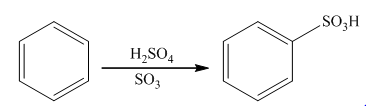
(c)
Interpretation:
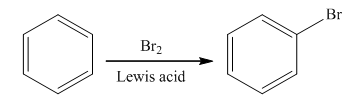
The main product of the monobromination of
Concept introduction:
The electrophilic aromatic substitution is the type of reaction in which an electrophile substitutes the hydrogen atom of benzene. A general electrophilic aromatic substitution reaction of benzene can be written as:
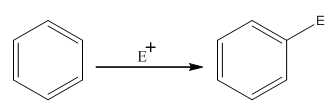
Where,
The activating groups are the groups that have the ability to donate the electron density to the benzene ring.
The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta direction groups are deactivating groups.
Halogenation is the
The bromination takes place in the presence of Lewis acid and bromine molecule. In this reaction, the bromonium ion is produced that acts as an electrophile. Brominium will attach on the benzene ring to form the final product. The general reaction is as follows:
Trending nowThis is a popular solution!

Chapter 27 Solutions
GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
- Show how to convert carboxylic acids to other functional groups, and devisemultistep syntheses using carboxylic acids as starting materials and intermediates.Explain how acid chlorides are used as activated derivatives of carboxylic acidsarrow_forward3b)Give the mechanisms for the following transformations:arrow_forwardOutline the step-by-step method (initiation, propagation(s), and one termination step) for bromination of 2-bromopropane to produce its major product.arrow_forward
- The ethylation of benzene is an electrophilic substitution reaction. Identify the electrophile in the reaction. Write the equation including reagents and conditions for the ethylation of benzene to produce ethylbenzene. Hence outline the mechanism for the reaction.arrow_forwardHow can 3,4-dimethyl-3-nitrohexane be synthesized from butane? Write a scheme for the sequential reactionarrow_forwardPredict the products, propose mechanisms for the reactions of carboxylic acidderivatives with reducing agents, alcohols, amines, and organometallic reagentssuch as Grignard, organolithium, and organocuprate reagentsarrow_forward
- Suggest mechanisms for the following reactionsarrow_forwardHow to synthesize 1-phenyl-1-bromopropane from benzene?arrow_forwardthe organic compound 2-heptanone, belonging to the ketone family, is responsible for the strong penetrating odor in Roquefort cheeses. Starting from acetylene as the starting reagent, propose a synthesis line with the reaction mechanisms involved for the synthetic obtaining of 2-heptanone and use it as a food additive in analogous cheeses.arrow_forward
- Enamines normally react with methyl iodide to give two products: one arising from alkylation at nitrogen and the second arising from alkylation at carbon. For example, Heating the mixture of C-alkylation and N-alkylation products gives only the product from C-alkylation. Propose a mechanism for this isomerization.arrow_forwardAldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forwardNonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

