
(a)
Interpretation:
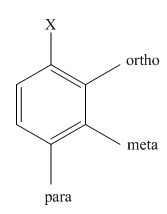
The product of the monobromination of
Concept introduction:
Halogenation is the
The activating groups are the groups that have the ability to donate the electron density to the benzene ring. The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta directing groups are deactivating groups.
The different positions with respect to group
(b)
Interpretation:
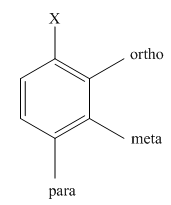
The product of the monobromination of aniline should be predicted.
Concept introduction:
Halogenation is the chemical reaction that involves the addition of one or more halogens to the compound. Bromination is the type of halogenations reaction that involves the addition of bromine to the compound. When bromine is added to a single position in the compound, the reaction is known as monobromination.
The activating groups are the groups that have the ability to donate the electron density to the benzene ring. The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta directing groups are deactivating groups.
The different positions with respect to group
(c)
Interpretation:
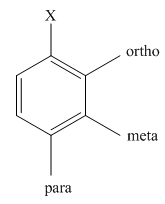
The product of the monobromination of
Concept introduction:
Halogenation is the chemical reaction that involves the addition of one or more halogens to the compound. Bromination is the type of halogenations reaction that involves the addition of bromine to the compound. When bromine is added to a single position in the compound, the reaction is known as monobromination.
The activating groups are the groups that have the ability to donate the electron density to the benzene ring. The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta directing groups are deactivating groups.
The different positions with respect to group
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
- Give the structural formula of A, B & C Give the reagents and reaction conditions for the steps labelled v, x, w & yarrow_forwardIn the reactions given below, write to which organic molecule Y, Z, T, A ‘belongs, and give information about the reaction types that take place.arrow_forwardYou are required to synthesize 2-bromopentane from the reaction between an alkene with HBr. Which alkene, 1-pentene or 2-pentene, should you react with HBr in order to get 2-bromopentane? Give an explanation.arrow_forward
- The rate law for addition of Br2 to an alkene is first orderin Br2 and first order in the alkene. Does this informationsuggest that the mechanism of addition of Br2 to analkene proceeds in the same manner as for addition of HBr?Explain.arrow_forwardGive a proposal on the synthesis methods of CoSO4.7H2Oarrow_forwardIsoprene has sometimes been used as a starting material in the laboratory synthesis of terpenes. In one such synthesis, the first step is the electrophilic addition of 2 mol of hydrogen bromide to isoprene to give 1,3-dibromo-3-methylbutane.Write a series of equations describing the mechanism of this reaction.arrow_forward
- Following are the steps in the industrial synthesis of glycerin. Provide structures for all intermediate compounds (AD) and describe the type of mechanism by which each is formed.arrow_forwardOn treatment with Cl2/hν, a compound with the formula C9H12 yields only a single monochloride. What is a possible structure of the compound?arrow_forwardThe formation of the following cyclic compound has been observed in the hydration of the following alkene, write a mechanism that explains the product.arrow_forward
- Drawing on what you know about the stereochemistry of alkene addition reactions, a. write the mechanism for the reaction of 2-butyne with one equivalent of Br2. b. predict the configuration of the product of the reaction.arrow_forwardThe major product formed when methylenecyclohexane is treated with NBS in dichloromethane is 1-(bromomethyl)cyclohexene. Account for the formation of this product.arrow_forwardSome 2-methyl-2-butene may be produced in the reaction as a by-product. Give a mechanism for its production.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
