
Concept explainers
The reduction of
The Grignard reagent is rarely isolated It is formed in solution and used immediately in the desired reaction. The alkylmetal bond is highly polar, with the partial negative charge on the C atom, which makes the C atom highly nucIeophilic The Grignard reagent ((R MgJ3r) ) can attack a carbonyl group in an aldehyde or ketone as follows:
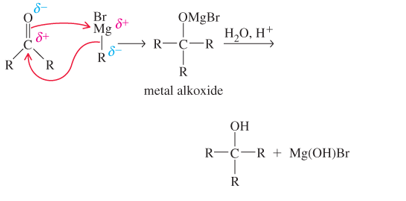
Addition of dilute aqueous acid solution to the metal alkoxide furnishes the alcohol. The important synthetic consequence of this procedure is that we have prepared a product with more carbon atoms than present in the starting material. A simple starting material can be transformed into a more complex molecule.
a. What is the product of the reaction between methanal and the Grignard reagent formed from 1-bromobutane after the addition of dilute acid?
b. By using a Grignard reagent, devise a synthesis for hexan-2-ol
c. By using a Grignard reagent, devise a synthesis for 2-methylhexan-2-ol. d. Grignard reagents can also be formed with aryl halides, such as chlorobenzene. What would be the product of the reaction between the Grignard reagent of chlorobenzene and propanone? Can you think of an alternative synthesis of this product, again using a Grignard reagent?
e. The basicity of the C atom bound to the magnesium in the Grignard reagent can be used to make Grignard reagents of terminal
f. By using a Grignard reagent, suggest a synthesis for hept-2-yn-1-oi
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
Additional Science Textbook Solutions
Chemistry
Thermodynamics, Statistical Thermodynamics, & Kinetics
Chemistry: The Central Science (13th Edition)
Chemistry: Structure and Properties
Chemistry
Principles of Chemistry: A Molecular Approach (3rd Edition)
- The addition of water to aldehydes and ketones occurs rapidly, although it is not thermodynamically favored. What would be the product for the reaction above? Hint: Think of the self-ionization of water and the polarity of the carbonyl group.arrow_forwardAlcohols are important for organic synthesis, especially in situations involving alkenes. The alcohol might be the desired product, or the OH group might be transformed into another functional group via halogenation, oxidation, or perhaps conversion to a sulfonic ester derivative. Formation of an alcohol from an alkene is particularly powerful because conditions can be chosen to produce either the Markovnikov or non-Markovnikov product from an unsymmetrical alkene. Using your reaction roadmap as a guide, show how to convert 4-methyl-1-pentene into 5-methylhexanenitrile. You must use 4-methyl-1-pentene and sodium cyanide as the source of all carbon atoms in the target molecule. Show all reagents needed and all molecules synthesized along the way.arrow_forwardWhen toluene is treated with sulfuric and nitric acids under special conditions, three nitro (NO2) groups are substituted for hydrogens at the 2, 4 and 6 positions on the ring (the next section discusses why the 2, 4, and 6 positions are substituted). The product is a highly explosive substance called 2,4,6-trinitrotoluene. This subastance is commonly known by a three letter name. What is it?arrow_forward
- Shown below is a carbocation intermediate in an electrophilic addition reaction of HCl with two different alkenes. Draw structural formulas for both of the alkenes.arrow_forwardHow is the mechanism for oxymercuration/demercuration reactions of alkenes understood? How are the (Markovnikov) products predicted and what reagents would you need to use to incorporate this reaction into a multistep synthesis strategy. Finally, what makes this a potentially more useful way to synthesize alcohols from alkenes than simple acid hydration?arrow_forwardIn the reaction (E) 2-methyl -2,4-hexadiene with hydrogen bromide at room temperature, two isomeric products are isolated. Draw the structures for these isomeric products. Which is the kinetic isomer and which is the thermodynamic isomer.arrow_forward
- Acetal derivatives of aldehydes and ketones are prepared by an acid-catalyzed dehydration reaction with alcohols or diols. Using more pictures than words, draw a reaction and a mechanism that shows the formation of acetal.arrow_forward1. What are the various ways by which alkenes may be synthesized?2. Give two examples each of Unsymmetrical alkenes and reagents.3. Give two examples of reactions of alkenes that result in Anti-Markonikov’s addition productsarrow_forwardplease explain how to synthesize the following molecule using only benzene or 2-carbon molecules. Show the mechanisms for each step ( including electron movement, bonds breaking and forming, curly arrows) and any reaction namesarrow_forward
- 1. i.What are the various ways by which alkenes may be synthesized? ii. Give two examples each of Unsymmetrical alkenes and reagents. iii. Give two examples of reactions of alkenes that result in Anti-Markonikov’s addition productsarrow_forwardBased on the characteristics of the carbonyl group (C = O), what reactions or transformations take place with aldehydes and ketones? a. nucleophilic additions by oxygenb. electrophilic additions by carbon attackc. nucleophilic additions by carbon attackd. electrophilic substitutions through a carbocationand. acid-base because carbonyl can act as both an electrophile and a nucleophilearrow_forwardGive the product and mechanism for the following reaction. Be sure to include all mechanism arrows, lone pairs, and formal charges in your mechanism. The product is an alkene.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning




