
Concept explainers
(a)
Interpretation:
The structure of the principal product obtained by the reaction between
Concept introduction:
Dichromate ion in acidic solution acts as a strong oxidizing agent. Primary alcohols upon oxidation produce
(b)
Interpretation:
The structure of the principal product obtained by the reaction between butyric acid and ethanol in an acidic solution should be drawn.
Concept introduction:
Esterification reaction is the
A general esterification reaction is given as follows:
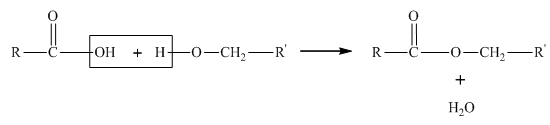
(c)
Interpretation:
The structure of the principal product obtained by the reaction between
Concept introduction:
Reaction of
Addition of hydrogen halide to unsymmetrical alkene occurs in accordance with Markovnikov’s rule. It states that addition of hydrogen halide to unsymmetrical alkene occurs in such a manner that hydrogen attaches to the carbon having more number of carbon and halide part goes to the carbon with less number of hydrogen atoms.
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
- What is the reaction from compound F to G? a. esterification b. hydrolysis c. oxidation d. reductionarrow_forwardA compound of molecular formula C5H10O forms a yellow precipitate with 2,4-dinitrophenylhydrazine reagent and a yellow precipitate with reagents for the iodoform test. a) Draw the structural formulae b) Name the two (2) compounds that fit these tests.arrow_forwardWhich of these could result to a higher product yield? a. Addition of 0.1M H2SO4 instead of concentrated H2SO4. b. Na2SO4 was not added in the obtained organic layer. c. Pure alcohols and carboxylic acids were used in the synthesis d. The refluxed solution was not purified.arrow_forward
- (a) Account for the following :(i) Cl – CH2COOH is a stronger acid than CH3COOH.(ii) Carboxylic acids do not give reactions of carbonyl group.(b) Write the chemical equations to illustrate the following name reactions:(i) Rosenmund reduction (ii) Cannizzaro’s reaction(c) Out of CH3CH2 – CO – CH2 – CH3 and CH3CH2 – CH2 – CO – CH3, which gives iodoform test?arrow_forward1. Illustrate the Synthesis or Preparation of Benzoic Acid from the oxidation of Benzaldehyde with Tollen's reagent. Instruction: a.) Write the Correct Structural Formula of the Reactant/s b.) Write the formula of the specific Catalyst/s of the reaction c.) Predict the Product/s of the given reactions.arrow_forwardReview the oxidation reactions using Cr6+reagents. Then draw the product formed when compound B is treated with following reagent. CrO3, H2SO4, H2Oarrow_forward
- Predict the products resulting from vigorous oxidation of compound by H2CrO4.arrow_forwardOn synthesis of esters via nucleophilic acyl substitution: Write the chemical equation involved in the reaction between the excess acid and NaHCO3. Given this, briefly explain why NaHCO3 is preferred over NaOH for the neutralization of excess acid. Also, explain how excess alcohol was eliminated from the crude product.arrow_forwardDraw the structure of the product of the reaction between the compound shown below and H2SO4.arrow_forward
- reaction that occurs when isopropyltriethylammoniumhydroxide is heatedarrow_forwardComplete the reaction: 42K19 → + β + [a]_____________arrow_forwardFour of the seven steps in the mechanism for this process are shown in the conversion of acetal A to hemiacetal E. a.) Add curved arrows for each step b.) Draw another resonance structure for C.c.) Identify the nucleophile and electrophile in Step [3].d.) Which steps are Brønsted–Lowry acid–base reactions?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
