
(a)
Interpretation:
The main products of mononitration of chlorobenzene should be predicted.
Concept introduction:
The electrophilic aromatic substitution is the type of reaction in which an electrophile substitutes the hydrogen atom of the
The nitration takes place in the presence of nitric acid and sulphuric acid. In this reaction, the protonation of nitric acid occurs in order to produce the nitronium ion.
The activating groups are the groups that have the ability to donate the electron density to the benzene ring. The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta directing groups are deactivating groups.
The different positions with respect to group
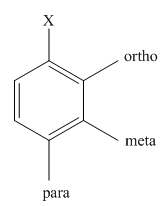
(b)
Interpretation:
The main products of monosulfonation of nitrobenzene should be predicted.
Concept introduction:
The electrophilic aromatic substitution is the type of reaction in which an electrophile substitutes the hydrogen atom of the aromatic compound. Sulphonation is an example of such a reaction.
The sulfonation takes place in the presence of sulphuric acid. In this reaction, sulfur trioxide is formed that acts as an electrophile.
The activating groups are the groups that have the ability to donate the electron density to the benzene ring. The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta directing groups are deactivating groups.
The different positions with respect to group
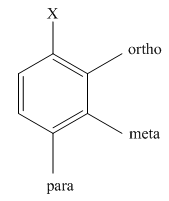
(c)
Interpretation:
The main products of monochlorination of
Concept introduction:
The electrophilic aromatic substitution is the type of reaction in which an electrophile substitutes the hydrogen atom of the aromatic compound. Chlorination is an example of such a reaction.
The chlorination takes place in the presence of aluminium chloride. In this reaction, the chloronium ion is produced that acts as an electrophile.
The activating groups are the groups that have the ability to donate the electron density to the benzene ring. The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta directing groups are deactivating groups.
The different positions with respect to group
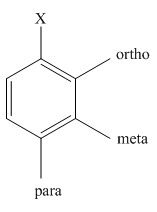
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
- When pent-2-yne reacts with mercuric sulfate in dilute sulfuric acid, the product is amixture of two ketones. Give the structures of these products, and use mechanisms toshow how they are formedarrow_forwardwrite the mechanism and predict the product, and includ the stereochemistryarrow_forward3. Obtain acetophenone and acetaldehyde by reaction of glycols with periodic acid. Justify your answer with the reaction mechanism.arrow_forward
- Establish the reaction and propose a mechanism for the reaction of benzene with bromine catalyzed with ferric bromidearrow_forwardWhat was the alkyne and the corresponding reagents for the synthesis of:arrow_forwardThe nitro groups on the benzene ring in the reactant serve two purposes.One is to let you know what atoms in the reactant correspond to what atomsin the product. But what role do the nitro groups play electronically – whywould the reaction be much slower if these nitro groups weren’t attached tothose benzene carbons? Draw any relevant structures to support youranswer.arrow_forward
- Complete the following mechanism for the acid-catalyzed rearrangement of 1-vinyl-2-phenylcyclopropane to give both isomers (E / Z) of 5-bromo-5-phenyl-2-pentene employing the curved-arrow/arrow-pushing formalism.arrow_forwardProvide Examples of dehydrohalogenation of dihalides to afford alkynes ?arrow_forwardShow how you would synthesize nitrobenzene from benzene, using a mixture of nitric acid and sulfuric acid. Write the overall reaction for the preparation of the electrophile and the mechanism for the substitution reactionarrow_forward
- Provide the structure of the two organic product(s) which results when 2-bromo-2-methylbutane is treated with sodium ethoxide.arrow_forwardGive a proposal on the synthesis methods of CoSO4.7H2Oarrow_forward(i) Name, draw and describe the organic product of the reaction between 2-methylbut-1-ene and H2O in the presence of H2SO4 and provide a clear rationale as to why this is the major product of the reaction. (ii) The elimination reaction between 2-bromobutane and NaOCH2CH3 gives two organic products. Draw a mechanism for the reaction which produces the major organic elimination product and provide a rationale as to why that is the major product.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
