
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 3, Problem 3.21QAP
Interpretation Introduction
Interpretation:
The location of the contact in a slide wire along its length for the circuit shown below should be identified.
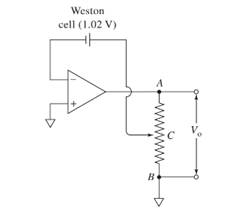
Concept introduction:
A voltage follower circuit is here with a Weston cell in the feedback loop. Since the op-amp has very high input resistance, the voltage from the Weston cell will appear across the output resistance part BC. The voltage across BC can be obtained using potential divider concept. Using this we can find the point at which the contact C is to be placed.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The purity of a sample of K3Fe(CN)6 is determined using hydrodynamic voltammetry at a glassy carbon electrode. The following data were obtained for a set of calibration standards.
A sample of impure K3Fe(CN)6 is prepared for analysis by diluting a 0.264 g portion to volume in a 100 mL volumetric flask. The limiting current for the sample is 444 μA.
a) What type of calibration method is used? (Internal Standard, External Standard, or Standard Addition?)
b) What is the purity of this sample of K3Fe(CN)6 (FW=329.25 g/mol)?
An atomic beam is designed to function with (a) cadmium, (b) mercury. The source is an oven maintained at 380K with a small slit of dimensions 1.0cm x 1.0 x 10-3cm. The vapor pressure of cadmium is 0.13 Pa nd that of mercury is 12 Pa at this temperature. What is the atomic current (the number of atoms per second) in the beams?
We apply a voltage of 10 V to Fcc an aluminum wire 2 mm diameter and 20 m long , if 10 % of the valence electrons carry the electrical charge , the parameter of cell α = 0. 4049 nm , electrical conductivity σ = 3.77 ×105 cm-1. Ω-1. , calculate the average drift velocity of the electrons in m/sec
Chapter 3 Solutions
Principles of Instrumental Analysis
Ch. 3 - Prob. 3.1QAPCh. 3 - Prob. 3.2QAPCh. 3 - Prob. 3.3QAPCh. 3 - Prob. 3.4QAPCh. 3 - Prob. 3.5QAPCh. 3 - Prob. 3.6QAPCh. 3 - Prob. 3.7QAPCh. 3 - Prob. 3.8QAPCh. 3 - Prob. 3.9QAPCh. 3 - Prob. 3.10QAP
Ch. 3 - Prob. 3.11QAPCh. 3 - Prob. 3.12QAPCh. 3 - Prob. 3.13QAPCh. 3 - Prob. 3.14QAPCh. 3 - Prob. 3.15QAPCh. 3 - Prob. 3.16QAPCh. 3 - Prob. 3.17QAPCh. 3 - Prob. 3.18QAPCh. 3 - Prob. 3.19QAPCh. 3 - Prob. 3.20QAPCh. 3 - Prob. 3.21QAPCh. 3 - Prob. 3.22QAPCh. 3 - Prob. 3.23QAPCh. 3 - Prob. 3.24QAPCh. 3 - Prob. 3.25QAP
Knowledge Booster
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

