
The Tolien’s test for the presence of reducing sugars (say, in a urine sample) involves treating the sample with silver ions in aqueous ammonia. The result is the formation of a silver mirror within the reaction vessel if a reducing sugar is present. Using glucose, C6H12O6, to illustrate this test, the
C6H12O6 (aq) + 2 Ag+(aq) + 2OH–(aq) → C6H12O7(aq) + 2 Ag(s) + H2O(ℓ) What has been oxidized, and what has been reduced? What is the oxidizing agent, and what is the reducing agent?
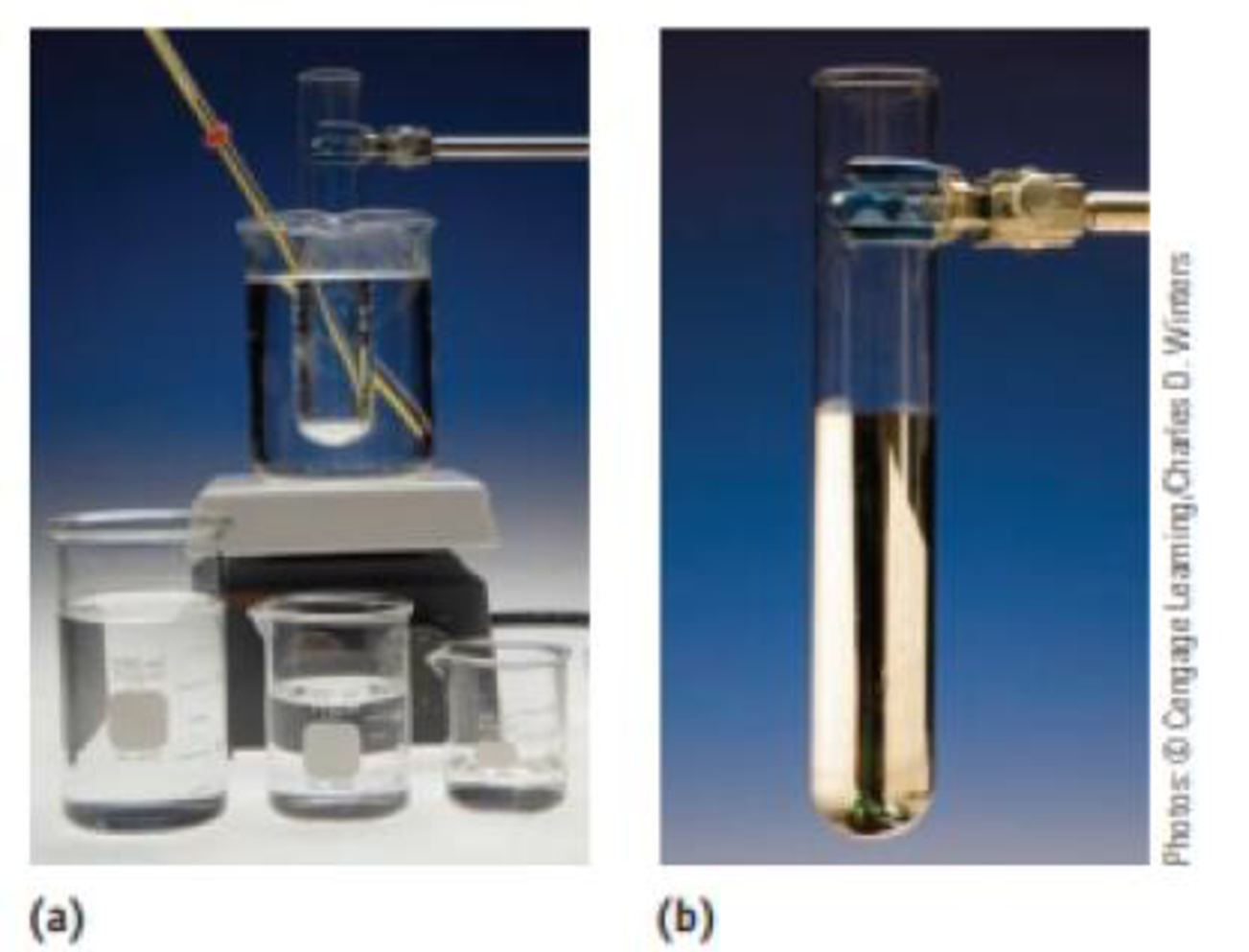
Tolien's test. The reaction of silver ions with a sugar such as glucose produces metallic silver. (a) The set-up for the reaction. (b) The silvered test tube
Trending nowThis is a popular solution!

Chapter 3 Solutions
Chemistry & Chemical Reactivity
- The iron content of hemoglobin is determined by destroying the hemoglobin molecule and producing small water-soluble ions and molecules. The iron in the aqueous solution is reduced to iron(II) ion and then titrated against potassium permanganate. In the titration, iron(ll) is oxidized to iron(III) and permanganate is reduced to manganese(II) ion. A 5.00-g sample of hemoglobin requires 32.3 mL of a 0.002100 M solution of potassium permanganate. The reaction with permanganate ion is MnO4(aq)+8H+(aq)+5Fe2+(aq)Mn2+(aq)+5Fe3+(aq)+4H2O What is the mass percent of iron in hemoglobin?arrow_forwardWrite balanced net ionic equations for the following reactions in acid solution. (a) Liquid hydrazine reacts with an aqueous solution of sodium bromate. Nitrogen gas and bromide ions are formed. (b) Solid phosphorus (P4) reacts with an aqueous solution of nitrate to form nitrogen oxide gas and dihydrogen phosphate (H2PO4-) ions. (c) Aqueous solutions of potassium sulfite and potassium permanganate react. Sulfate and manganese(II) ions are formed.arrow_forwardThe Ostwald process for the commercial production of nitric acid involves the Following three steps: 4NH3(g)+5O2(g)4NO(g)+6H2O(s)2NO(g)+O2(g)2NO2(g)3NO2(g)+H2O(l)2HNO3(aq)+NO(g) a. Which reaction in the Ostwald process are oxidation-reduction reactions? b. Identify each oxidizing agent and reducing agent.arrow_forward
- Gold can be dissolved from gold-bearing rock by treating the rock with sodium cyanide in the presence of oxygen. 4 Au(s) + 8 NaCN(aq) + O2(g) + 2 H2O() 4 NaAu(CN)2(aq) + 4 NaOH(aq) (a) Name the oxidizing and reducing agents in this reaction. What has been oxidized, and what has been reduced? (b) If you have exactly one metric ton (1 metric ton = 1000 kg) of gold-bearing rock, what volume of 0.075 M NaCN, in liters, do you need to extract the gold if the rock is 0.019% gold?arrow_forwardAn electrolytic cell is set up with Cd(s) in Cd(NO3)2(aq) and Zn(s) in Zn(NO3)2(aq). Initially both electrodesweigh 5.00 g. After running the cell for several hours theelectrode in the left compartment weighs 4.75 g. (a) Which electrode is in the left compartment? (b) Does the mass of the electrode in the right compartmentincrease, decrease, or stay the same? If the masschanges, what is the new mass? (c) Does the volume of the electrode in the right compartment increase, decrease, or stay the same? If the volumechanges, what is the new volume? (The density of Cd is8.65 g/cm3.)arrow_forwardXenon trioxide, XeO3, reacts with aqueous base to form the xenate anion, HXeO4. This ion reacts further with OH to form the perxenate anion, XeO64, in the following reaction: 2HXeO4(aq)+2OH(aq)XeO64(aq)+Xe(g)+O2(g)+2H2O(l) Identify the elements that are oxidized and reduced in this reaction. You will note that the equation is balanced with respect to the number of atoms on either side. Verify that the redox part of this equation is also balanced, that is, that the extents of oxidation and reduction are also equal.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





