
Concept explainers
(a)
Interpretation:
The acid-base reaction should be described between the following reactant by using curved arrows and each curved arrow having positive or negative should be marked. The value of

Concept Introduction:
A substance which produces
A substance which produces hydroxide ion when dissolved in aqueous solution is known as a base. These are proton acceptor.
A
(b)
Interpretation:
The acid-base reaction should be described between the following reactant by using curved arrows and each curved arrow having positive or negative should be marked. The value of

Concept Introduction:
A substance which produces
A substance which produces hydroxide ion when dissolved in aqueous solution is known as a base. These are proton acceptor.
A chemical reaction which takes place between an acid and a base is known as acid-base reaction.
(c)
Interpretation:
The acid-base reaction should be described between the following reactant by using curved arrows and each curved arrow having positive or negative should be marked. The value of
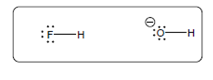
Concept Introduction:
A substance which produces
A substance which produces hydroxide ion when dissolved in aqueous solution is known as a base. These are proton acceptor.
A chemical reaction which takes place between an acid and a base is known as acid-base reaction.
(d)
Interpretation:
The acid-base reaction should be described between the following reactant by using curved arrows and each curved arrow having positive or negative should be marked. The value of
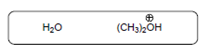
Concept Introduction:
A substance which produces
A substance which produces hydroxide ion when dissolved in aqueous solution is known as a base. These are proton acceptor.
A chemical reaction which takes place between an acid and a base is known as acid-base reaction.
(d)
Interpretation:
The acid-base reaction should be described between the following reactant by using curved arrows and each curved arrow having positive or negative should be marked. The value of
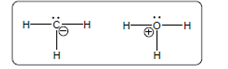
Concept Introduction:
A substance which produces
A substance which produces hydroxide ion when dissolved in aqueous solution is known as a base. These are proton acceptor.
A chemical reaction which takes place between an acid and a base is known as acid-base reaction.
(d)
Interpretation:
The acid-base reaction should be described between the following reactant by using curved arrows and each curved arrow having positive or negative should be marked. The value of
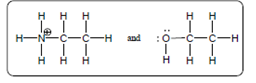
Concept Introduction:
A substance which produces
A substance which produces hydroxide ion when dissolved in aqueous solution is known as a base. These are proton acceptor.
A chemical reaction which takes place between an acid and a base is known as acid-base reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry: A Guided Inquiry
- In the following reaction, which is the acid, which is the base, which is the conjugate acid and which is the conjugate base C2H- + CH3OH = CH3O- + C2H2arrow_forwardAt equilibrium, the value of [H+] in a 0.260M solution of an unknown acid is 0.00418M . Determine the degree of ionization and the Ka of this acid.arrow_forwardIn the attached reactions how can I tell if they are: A BASE or Conjugate Acidarrow_forward
- Complete the following Conjugate equation. NH4+ - H+ ------>arrow_forwardIn acid base reactions: stronger acid + stronger base --> weaker acid + weaker base Given this reaction: H2O + H2O <--> H3O+ + –OH Predict whether it: goes to the left, towards reactants or goes to the right, towards products Please explain your choice.arrow_forwardWhat is the acid dissociation constant (Ka) expression for the following reaction? CH3COOH+H2O=CH3COO-+H3Oarrow_forward
- Draw the products of the following acid-base reaction, and determine if equilibrium favors reactants or products. Be sure to include counter ions.arrow_forwardI'm having hard time understanding this question can you please help show how to approach this probelm?arrow_forwardi am still not seeing why. whats a rule i can adopt to understand which proton to select in a situation like thisarrow_forward
- Hi! I need help with these! The topic for this is Curved Arrows/ Acid-Base reactions.arrow_forwardFor the following molecules, identify the basic electron pairs. For each basic electron pair that you identify, draw the conjugate acid that results from that basic electron pair reacting with a proton (H+). For molecules with more than one basic electron pair, draw a separate conjugate acid for each onearrow_forwardUsing the acid dissociation reaction find the Ka expressionarrow_forward
