
(a)
Interpretation:The molecular formula for the given molecule needs to be determined.
Concept Introduction: The molecular formula of any compound tells the number of atoms of different elements present in the molecule.
(a)
Answer to Problem SII1RE
Covalent bonds are formed by sharing of equal number of electrons. Molecular structure of any compound represents the presence of different atoms in a compound.
Explanation of Solution
The structure of the given compound is as follows:
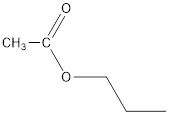
This, the formula will be CH3COOCH2CH2CH3or C5H10O2(Propyl ethanoate)
(b)
Interpretation: The Lewis dot structure of the molecule needs to be drawn.
Concept Introduction: Every element completes its octet to form bonds with different atoms.
(b)
Explanation of Solution
Lewis dot structure can be drawn with the help of valence electrons. Carbon has 4 valence electrons, oxygen has 6 valence electrons. To complete the octet, carbon will share 4 electrons, two electrons each with oxygen and form double covalent bond.
The given compound is as follows:
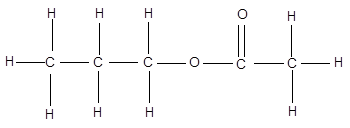
The Lewis dot structure will be:
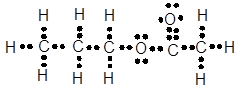
(C)
Interpretation: The
Concept Introduction: In a compound, functional group is a specific group which represents the chemical properties of the compound.
(C)
Explanation of Solution
Functional groups of different compounds have specific bonding between different atoms.
Ester group (- COOR ) Functional group is present in propyl acetate.
(d)
Interpretation: The smell of the compound needs to be predicted.
Concept Introduction: Specific functional group has specific smell,thus, different compounds have different type of smells.
(d)
Answer to Problem SII1RE
Ester group compounds are generally sweet smelling.
Explanation of Solution
Ester (- COOR ) functional group is present in propyl acetate. It is a colorless liquid having a characteristic sweet smell like pear. It is used in nail paint removers, as a solvent, etc.
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry: A Molecular Approach (4th Edition)
Chemistry: The Central Science (13th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Chemistry: The Central Science (14th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





