
Concept explainers
(a)
Interpretation:
The Lewis dot structure for TeCl2 must be drawn.
Concept Introduction :
Lewis dot structure is the representation of a molecule using valence electrons shown as dots.
Te is a p block element of group 16 with 6 valence electrons.
(a)
Answer to Problem 5E
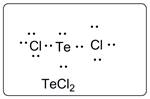
Lewis dot structure for TeCl2 is given below.
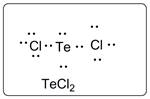
.
Explanation of Solution
In TeCl2, Te is the central atom which is bonded to two Cl atoms. There are three lone pairs of electrons on each Cl atom and two lone pair of electrons on Te atom.
Thus, the Lewis dot structure is as follows:
(b)
Interpretation:
The Lewis dot structure for HI must be drawn.
Concept Introduction :
Lewis dot structure is the representation of a molecule using valence electrons shown as dots.
I is a p block element of group 17 with 7 valence electrons.
(b)
Answer to Problem 5E
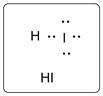
Lewis dot structure for HI is given below.
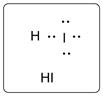
.
Explanation of Solution
In HI, I is bonded to one H atom. There are three lone pairs of electrons on I atom and hydrogen has zero lone pairs.
The structure is represented as follows:
(c)
Interpretation:
Lewis dot structures for AsBr3 must be drawn.
Concept Introduction :
Lewis dot structure is the representation of a molecule using valence electrons shown as dots.
As is a p block element of group 15 with 5 valence electrons.
(c)
Answer to Problem 5E
Lewis dot structure for AsBr3 is given below.
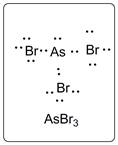
.
Explanation of Solution
In AsBr3, As is the central atom which is bonded to three Br atoms. There are one lone pair of electrons on As atom and three lone pairs of electrons on Br atom.
The structure is represented as follows:
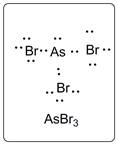
(d)
Interpretation:
Lewis dot structures for SiF4 must be drawn.
Concept Introduction :
Lewis dot structure is the representation of a molecule using valence electrons shown as dots.
Si is a p block element of group 14 with 4 valence electrons.
(d)
Answer to Problem 5E
Lewis dot structure for SiF4 is given below.
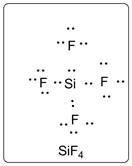
.
Explanation of Solution
In SiF4, Si is the central atom which is bonded to four F atoms. There are three lone pairs of electrons on F atom.
Structure is represented as follows:
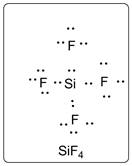
(e)
Interpretation:
Lewis dot structures for F2 must be drawn.
Concept Introduction :
Lewis dot structure is the representation of a molecule using valence electrons shown as dots.
F is a p block element of group 17 with 7 valence electrons.
(e)
Answer to Problem 5E
Lewis dot structure for F2 is given below.
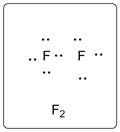
.
Explanation of Solution
In F2, both the F atoms are bonded to each other with a single bond. There are three lone pairs of electrons on each F atom.
The structure is represented as follows:
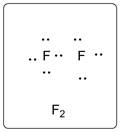
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Chemistry: Matter and Change
Organic Chemistry (8th Edition)
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry: A Molecular Approach
Chemistry For Changing Times (14th Edition)
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





