
Concept explainers
(a)
Interpretation:
The isomer of given compound needs to be drawn.
Concept introduction:
Isomers can be defined as the organic molecules which have the same molecular formula and different structural formula.
Due to different structural formula, isomers have different physical and chemical properties.
Answer to Problem 7E
Explanation of Solution
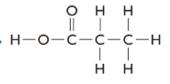
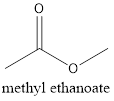
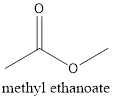
The formula of the given compound is CH3CH2COOH (propanoic acid). Propanoic acid is a carboxylic acid with two carbon atoms and a −COOH as a functional group. The functional isomer of propanoic acid will be methyl ethanoate which is an ester with general chemical formula as RCOOR.
The structure is represented as follows:
(b)
Interpretation:
The isomer of a given compound needs to be determined.
Concept introduction:
Isomers can be defined as the organic molecules which have the same molecular formula and different structural formula.
Due to different structural formula, isomers have different physical and chemical properties.
Answer to Problem 7E

Explanation of Solution
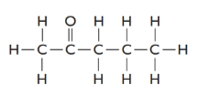
The given structure is as follows:
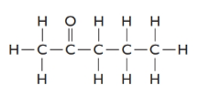
There are 5 carbon atoms thus, it is 2-pentanone.
2-pentanone is an isomer of pentanal as it also contains 5 carbon atoms, 1 O atom and 10 H atoms.
The structure of pentanal is represented as follows:

(c)
Interpretation:
The isomer of a given compound needs to be determined.
Concept introduction:
Isomers can be defined as the organic molecules which have the same molecular formula and different structural formula.
Due to different structural formula, isomers have different physical and chemical properties.
Answer to Problem 7E

Explanation of Solution
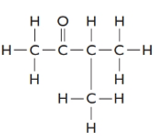
The given structure is as follows:
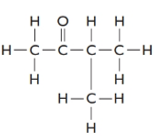
The formula of the given compound is
3-methylbutanone must be an isomer of pentanal as both have the same molecular formula but different structural formulas.
The structure of pentanal is as follows:

(d)
Interpretation:
The isomer of a given compound needs to be determined.
Concept introduction:
Isomers can be defined as the organic molecules which have the same molecular formula and different structural formula.
Due to different structural formula, isomers have different physical and chemical properties.
Answer to Problem 7E
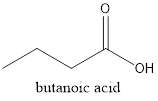
Explanation of Solution
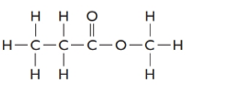
The given structure is as follows:
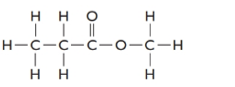
The formula of the given compound is
Methylpropanoate is an ester as RCOOR is the general formula. Esters are isomers of carboxylic acid therefore it must be carboxylic acid with 4 C atoms that is butanoic acid.
The structure is represented as follows:
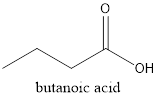
(e)
Interpretation:
The isomer of a given compound needs to be determined.
Concept introduction:
Isomers can be defined as the organic molecules which have the same molecular formula and different structural formula.
Due to different structural formula, isomers have different physical and chemical properties.
Answer to Problem 7E
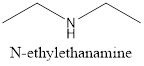
Explanation of Solution
The given structure is as follows:
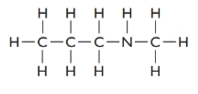
The formula of the given compound is
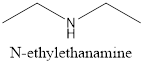
(f)
Interpretation:
The isomer of given compound needs to be determined.
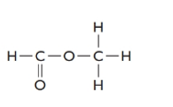
Concept introduction:
Isomers can be defined as the organic molecules which have the same molecular formula and different structural formula.
Due to different structural formula, isomers have different physical and chemical properties.
Answer to Problem 7E
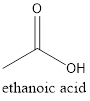
Explanation of Solution
The given structure is as follows:
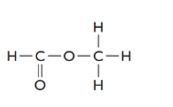
The formula of the given compound is
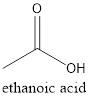
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Introductory Chemistry (5th Edition) (Standalone Book)
Organic Chemistry (8th Edition)
General Chemistry: Atoms First
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry For Changing Times (14th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





