
Concept explainers
(a)
Interpretation: Comprehend the orientation of methanol molecule towards a negatively charged wand.
Concept Introduction: The polarity of molecule depends on the presence of electropositive and electronegative atoms present in the molecule.
Due to electronegativity difference between bonded atoms, partial charges induce on the bonded atoms. The partial charges effect the physical properties of the polar molecules.
(a)
Answer to Problem 4E
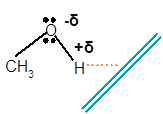
Explanation of Solution
Methanol is a polar molecule as it contains one −OH group. Here O is more electronegative than H atom. Therefore the −OH group is polar with partial negative charge on O and partial positive charge on H atom.
The molecule of methanol will orient towards a negative charge wand in such a way that the electropositive H atom will orient toward wand and the O atom will orient away from wand as it carries partial negative charge as given below:
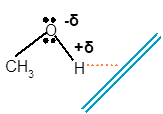
(b)
Interpretation: Comprehend the position of methanol molecules on waxed paper; either bead up or spread out.
Concept Introduction: The polarity of molecule depends on the presence of electropositive and electronegative atoms present in the molecule.
Due to electronegativity difference between bonded atoms, partial charges induce on the bonded atoms. The partial charges effect the physical properties of the polar molecules.
(b)
Answer to Problem 4E
Methanol molecules bead up over waxed paper.
Explanation of Solution
Methanol is a polar molecule as it contains one −OH group. Here O is more electronegative than H atom. Therefore the −OH group is polar with partial negative charge on O and partial positive charge on H atom. A polar molecule is always soluble in polar solvent. Wax is a non-polar compound as it is composed of long fatty acid chain.
Therefore methanol is not soluble in wax and methanol molecules will bead up over waxed paper.
(c)
Interpretation: Comprehend the solubility of methanol in water.
Concept Introduction: The polarity of molecule depends on the presence of electropositive and electronegative atoms present in the molecule.
Due to electronegativity difference between bonded atoms, partial charges induce on the bonded atoms. The partial charges effect the physical properties of the polar molecules.
(c)
Answer to Problem 4E
Methanol is soluble in water.
Explanation of Solution
Methanol is a polar molecule as it contains one −OH group. Here O is more electronegative than H atom. Therefore the −OH group is polar with partial negative charge on O and partial positive charge on H atom. A polar molecule is always soluble in polar solvent. Water is a polar molecule as it has partially negative O atom that is bonded with partially positive H atom.
Therefore methanol will be soluble in polar water as solvent.
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Chemistry (7th Edition)
Chemistry
Chemistry For Changing Times (14th Edition)
Essential Organic Chemistry (3rd Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Organic Chemistry (8th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





