
Concept explainers
(a)
Interpretation:
The structure of the given compound needs to be drawn using wedge, dashed and solid lines to represent the three-dimensional
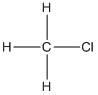
Concept Introduction:
Wedge and dash method is used to represent the three-dimensional structure of any molecule in which solid lines  represents the bonds in plane of the paper, wedge lines
represents the bonds in plane of the paper, wedge lines 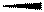 represents the bonds above the plane of the paper or towards the viewer and dashed lines
represents the bonds above the plane of the paper or towards the viewer and dashed lines 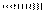 represents the bonds below the plane of the paper or away from the viewer.
represents the bonds below the plane of the paper or away from the viewer.
(b)
Interpretation:
The structure of the given compound needs to be drawn using wedge, dashed and solid lines to represent the three-dimensional
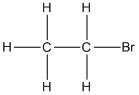 Concept Introduction:
Concept Introduction:
Wedge and dash method is used to represent the three-dimensional structure of any molecule in which solid lines  represents the bonds in plane of the paper, wedge lines
represents the bonds in plane of the paper, wedge lines 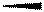 represents the bonds above the plane of the paper or towards the viewer and dashed lines
represents the bonds above the plane of the paper or towards the viewer and dashed lines 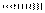 represents the bonds below the plane of the paper or away from the viewer.
represents the bonds below the plane of the paper or away from the viewer.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





