
Chemistry
9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12, Problem 83AE
Consider the following representation of the reaction 2NO2(g) → 2NO(g) + O2(g).
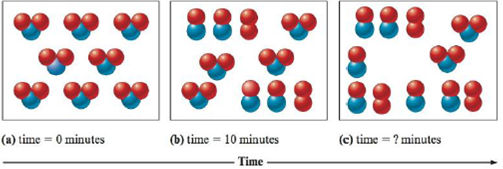
Determine the time for the final representation above if the reaction is
a. first order
b. second order
c. zero order
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 12 Solutions
Chemistry
Ch. 12 - Define reaction rate. Distinguish between the...Ch. 12 - Distinguish between the differential rate law and...Ch. 12 - One experimental procedure that can be used to...Ch. 12 - The initial rate for a reaction is equal to the...Ch. 12 - Consider the zero-, first-, and second-order...Ch. 12 - Derive expressions for the half-life of zero-,...Ch. 12 - Prob. 7RQCh. 12 - What two requirements must be met to call a...Ch. 12 - Prob. 9RQCh. 12 - Give the Arrhenius equation. Take the natural log...
Ch. 12 - Why does a catalyst increase the rate of a...Ch. 12 - Define stability from both a kinetic and...Ch. 12 - Describe at least two experiments you could...Ch. 12 - Make a graph of [A] versus time for zero-, first-,...Ch. 12 - How does temperature affect k, the rate constant?...Ch. 12 - Consider the following statements: In general, the...Ch. 12 - For the reaction A+BC, explain at least two ways...Ch. 12 - A friend of yours states, A balanced equation...Ch. 12 - Provide a conceptual rationale for the differences...Ch. 12 - The rate constant (k) depends on which of the...Ch. 12 - Each of the statements given below is false....Ch. 12 - Define what is meant by unimolecular and...Ch. 12 - The plot below shows the number of collisions with...Ch. 12 - For the reaction O2(g)+2NO(g)2NO2(g) the observed...Ch. 12 - The rate law for a reaction can be determined only...Ch. 12 - Table 12.2 illustrates how the average rate of a...Ch. 12 - The type of rate law for a reaction, either the...Ch. 12 - The initial rate of a reaction doubles as the...Ch. 12 - Hydrogen reacts explosively with oxygen. However,...Ch. 12 - The central idea of the collision model is that...Ch. 12 - Consider the following energy plots for a chemical...Ch. 12 - Prob. 21QCh. 12 - Would the slope of a ln(k) versus 1/T plot (with...Ch. 12 - Consider the reaction 4PH3(g)P4(g)+6H2(g) If, in a...Ch. 12 - In the Haber process for the production of...Ch. 12 - At 40C, H2O2 (aq) will decompose according to the...Ch. 12 - Consider the general reaction aA+bBcC and the...Ch. 12 - What are the units for each of the following if...Ch. 12 - The rate law for the reaction...Ch. 12 - The reaction 2NO(g)+Cl2(g)2NOCl(g) was studied at...Ch. 12 - The reaction 2I-(aq)+S2O82-(aq)I2(aq)+2SO42-(aq)...Ch. 12 - The decomposition of nitrosyl chloride was...Ch. 12 - The following data were obtained for the gas-phase...Ch. 12 - The reaction I(aq)+OCl(aq)IO(aq)+Cl(aq) was...Ch. 12 - The reaction 2NO(g)+O2(g)2NO2(g) was studied. and...Ch. 12 - The rote of the reaction between hemoglobin (Hb)...Ch. 12 - The following data were obtained for the reaction...Ch. 12 - The decomposition of hydrogen peroxide was...Ch. 12 - A certain reaction has the following general form:...Ch. 12 - The rate of the reaction NO2(g)+CO(g)NO(g)+CO2(g)...Ch. 12 - A certain reaction has the following general form:...Ch. 12 - The decomposition of ethanol (C2H5OH) on an...Ch. 12 - At 500 K in the presence of a copper surface,...Ch. 12 - The dimerization of butadiene 2C4H6(g)C8H12(g) was...Ch. 12 - The rate of the reaction O(g)+NO2(g)NO(g)+O2(g)...Ch. 12 - Experimental data for the reaction A2B+C have been...Ch. 12 - Prob. 46ECh. 12 - The reaction AB+C is known to be zero order in A...Ch. 12 - The decomposition of hydrogen iodide on finely...Ch. 12 - Prob. 49ECh. 12 - A first-order reaction is 75.0% complete in 320....Ch. 12 - The rate law for the decomposition of phosphine...Ch. 12 - DDT (molar mass = 354.49 g/mol) was a widely used...Ch. 12 - Consider the following initial rate data for the...Ch. 12 - The rate law for the reaction...Ch. 12 - Prob. 55ECh. 12 - Theophylline is a pharmaceutical drug that is...Ch. 12 - You and a coworker have developed a molecule...Ch. 12 - Consider the hypothetical reaction A+B+2C2D+3E...Ch. 12 - Write the rate laws for the following elementary...Ch. 12 - A possible mechanism for the decomposition of...Ch. 12 - A proposed mechanism for a reaction is...Ch. 12 - The mechanism for the gas-phase reaction of...Ch. 12 - For the following reaction profile, indicate a....Ch. 12 - Draw a rough sketch of the energy profile for each...Ch. 12 - The activation energy for the reaction...Ch. 12 - The activation energy for some reaction...Ch. 12 - The rate constant for the gas-phase decomposition...Ch. 12 - The reaction (CH3)3CBr+OH(CH3)3COH+Br in a certain...Ch. 12 - The activation energy for the decomposition of...Ch. 12 - A first-order reaction has rate constants of 4.6 ...Ch. 12 - A certain reaction has an activation energy of...Ch. 12 - Prob. 72AECh. 12 - Which of the following reactions would you expect...Ch. 12 - Prob. 74AECh. 12 - One mechanism for the destruction of ozone in the...Ch. 12 - One of the concerns about the use of Freons is...Ch. 12 - Assuming that the mechanism for the hydrogenation...Ch. 12 - The decomposition of NH3 to N2 and H2 was studied...Ch. 12 - The decomposition of many substances on the...Ch. 12 - Prob. 80AECh. 12 - A popular chemical demonstration is the magic...Ch. 12 - Prob. 82AECh. 12 - Consider the following representation of the...Ch. 12 - The reaction H2SeO3(aq) + 6I-(aq) + 4H+(aq) Se(s)...Ch. 12 - Consider two reaction vessels, one containing A...Ch. 12 - Sulfuryl chloride (SO2Cl2) decomposes to sulfur...Ch. 12 - For the reaction 2N2O5(g)4NO2(g)+O2(g) the...Ch. 12 - Experimental values for the temperature dependence...Ch. 12 - Cobra venom helps the snake secure food by binding...Ch. 12 - Iodomethane (CH3I) is a commonly used reagent in...Ch. 12 - Experiments during a recent summer on a number of...Ch. 12 - The activation energy of a certain uncatalyzed...Ch. 12 - Consider the reaction 3A+B+CD+E where the rate law...Ch. 12 - The thiosulfate ion (S2O32) is oxidized by iodine...Ch. 12 - The reaction A(aq)+B(aq)products(aq) was studied,...Ch. 12 - A certain substance, initially present at 0.0800...Ch. 12 - A reaction of the form aAProducts gives a plot of...Ch. 12 - A certain reaction has the form aAProducts At a...Ch. 12 - Prob. 99CWPCh. 12 - Consider the hypothetical reaction A2(g) + B2(g) ...Ch. 12 - Experiments have shown that the average frequency...Ch. 12 - Consider a reaction of the type aA products, in...Ch. 12 - A study was made of the effect of the hydroxide...Ch. 12 - Two isomers (A and B) of a given compound dimerize...Ch. 12 - The reaction NO(g)+O3NO2(g)+O2(g) was studied by...Ch. 12 - Most reactions occur by a series of steps. The...Ch. 12 - You are studying the kinetics of the reaction...Ch. 12 - The decomposition of NO2(g) occurs by the...Ch. 12 - The following data were collected in two studies...Ch. 12 - Consider the following hypothetical data collected...Ch. 12 - Consider the hypothetical reaction A+B+2C2D+3E In...Ch. 12 - Hydrogen peroxide and the iodide ion react in...Ch. 12 - Sulfuryl chloride undergoes first-order...Ch. 12 - Upon dissolving InCl(s) in HCl, In+(aq) undergoes...Ch. 12 - The decomposition of iodoethane in the gas phase...Ch. 12 - Consider the following reaction: CH3X+YCH3Y+X At...
Additional Science Textbook Solutions
Find more solutions based on key concepts
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
For Practice 1.1
Is each change physical or chemical? Which kind of property (chemical or physical) is demonst...
Principles of Chemistry: A Molecular Approach (3rd Edition)
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
Real walls are never totally adiabatic. Use your experience to order the following walls in increasing order wi...
Thermodynamics, Statistical Thermodynamics, & Kinetics
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Derive an expression for the half-life of a a third order reaction;b a reaction whose order is =1; c a reaction whose order is 12. In these last two cases, examples are rare but known.arrow_forwardThe decomposition of sulfuryl chlorideSO2Cl2fur dioxide and chlorine gases is a first-order reaction. It is found that at a certain temperature, it takes 1.43 hours to decompose 0.0714 M to 0.0681 M. (a) What is the rate constant for the decomposition? (b) What is the rate of decompostion [ SO2Cl2 ]=0.0462M? (c) How long will it take to decompose SO2Cl2 so that 45% remains?arrow_forwardWhich of the following statement(s) is( are) true? a. The half-life for a zero-order reaction increases as the reaction proceeds. b. A catalyst does not change the value of the rate constant c. The half-life for a reaction, aAProducts that is first order in A increases with increasing [A]0. d. The half-life for a second-order reaction increases as the reaction proceeds.arrow_forward
- We know that the decomposition of SO2Cl2 is first-order in SO2Cl2, SO2Cl2 SO2(g) + Cl2(g) with a half-life of 245 minutes at 600 K. If you begin with a partial pressure of SO2Cl2 of 25 mm Hg in a 1.0-L flask what is the partial pressure of each reactant and product after 245 minutes? What is the partial pressure of each reactant and product after 12 hours?arrow_forwardConsider a hypothetical reaction between A and B: A + B products Use the following initial rate data to calculate the rate constant for this reaction. [A] (mol/L) [B] (mol/L) Initial Rate (mol/L s) 0.20 1.0 3.0 0.50 1.0 11.8 2.0 2.0 189.5arrow_forwardA study of the rate of dimerization of C4H6 gave the data shown in the table: 2C4H6C8H12 (a) Determine the average rate of dimerization between 0 s and 1600 s, and between 1600 s and 3200 s. (b) Estimate the instantaneous rate of dimerization at 3200 s from a graph of time versus [C4H6]. What are the units of this rate? (c) Determine the average rate of formation of C8H12 at 1600 s and the instantaneous rate of formation at 3200 s from the rates found in parts (a) and (b).arrow_forward
- Consider the hypothetical first-order reaction 2A(g)X(g)+12Y(g)At a certain temperature, the half-life of the reaction is 19.0 min. A 1.00-L flask contains A with a partial pressure of 622 mm Hg. If the temperature is kept constant, what are the partial pressures of A, X, and Y after 42 minutes?arrow_forwardAt 620. K butadiene dimerizes at a moderate rate. The following data were obtained in an experiment involving this reaction: t(s) [C4H6] (mol/L) 0 0.01000 1000.. 0.00629 2000. 0.00459 3000. 0.00361 a. Determine the order of the reaction in butadiene. b. In how many seconds is the dimerization 1.0% complete? c. In how many seconds is the dimerization 10.0% complete? d. What is the half-life for the reaction if the initial concentration of butadiene is 0.0200 M? e. Use the results from this problem and Exercise 45 to calculate the activation energy for the dimerization of butadiene.arrow_forwardThe thermal decomposition of diacetylene, C4H2, was studied at 950 C. Use the following data (K. C. Hou and H. B. Palmer, Journal of Physical Chemistry. Vol. 60, p. 858, 1965) to determine the order of the reaction.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Kinetics: Initial Rates and Integrated Rate Laws; Author: Professor Dave Explains;https://www.youtube.com/watch?v=wYqQCojggyM;License: Standard YouTube License, CC-BY