
Chemistry In Context
9th Edition
ISBN: 9781259638145
Author: Fahlman, Bradley D., Purvis-roberts, Kathleen, Kirk, John S., Bentley, Anne K., Daubenmire, Patrick L., ELLIS, Jamie P., Mury, Michael T., American Chemical Society
Publisher: Mcgraw-hill Education,
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.2, Problem 12.7YT
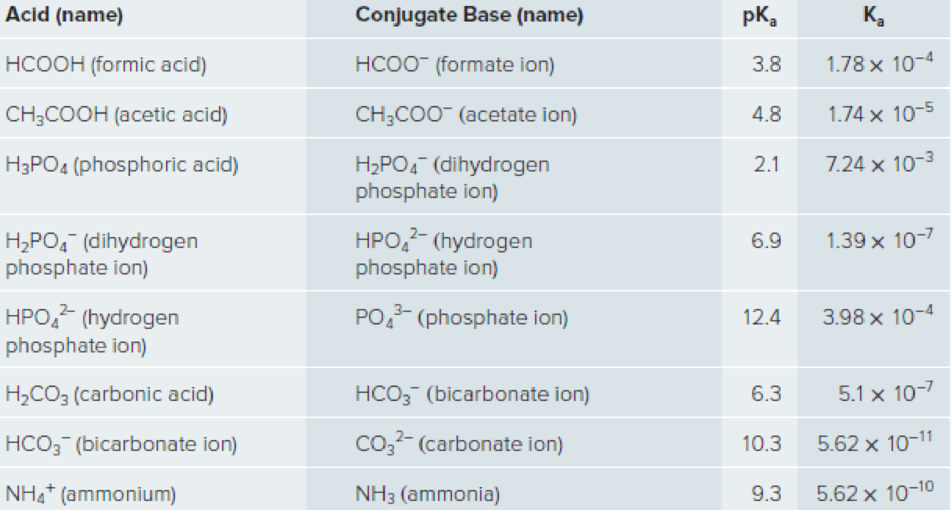
A practicing scientist must Judge a potential buffer for its utility in mimicking different cellular environments. Consider the list of target pH values and match them to the best possible chemical mixture for creating that pH, see Table 12.1 for a list.
- a. pH 2.5
- b. pH 7.4
- c. pH 6.8
- d. pH 5.4
Table 12.1 Dissociation of Some Weak Acids with Ka and pKa Values
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Part 1)A buffer solution contains 0.125 M acetic acid and 0.150 M sodium acetate. The pKa of acetic acid is 4.74. Use the Henderson-Hasselbalch equation to calculate the solution’s pH.
Part 2)A buffer solution is prepared by mixing 2.00 ml of 0.500 M acetic acid and 10.00 ml of 0.500 M sodium acetate. Calculate the acetic acid concentration in the buffer solution. You will need to use dilution calculations.
If to 100 ml of a solution of CH3COOH with a concentration of 0.0928 N.
a) 200 ml of distilled water was added. Determine its new concentration expressed to 4 decimal places, and determine the pH of the final solution. Data: pKa = 4.756 at 25°C. Consider that degree of dissociation is very small.
b) If it is mixed with 100 ml of a 0.0989 N CH3COONa solution. What is the pH of the buffer solution.
1. A buffer solution is prepared by taking 0.25 moles of acetic acid (pKa = 4.76) and 0.400 moles of barium acetate in sufficient water to make 1.400 liters of solution. Calculate the pH of this solution.
a. 4.25
b. 4.45
c. 5.00
d. 5.27
e. 5.35
2. You have 500.0 mL of a buffer solution containing 0.30M acetic acid and 0.20M sodium acetate. What will be the pH of this solution after the addition of 20.0mL of 1.00M HCl solution? (Ka = 1.8 x 10^ -5).
a. 4.74
b. 5.07
c. 4.00
d. 3.02
e. 4.42
3. 40.0mL of 0.10M HCl was added to 50.0 mL of 0.10M NaOH and the mixture was stirred, then tested with a pH meter. What is its pH at 25.0 C?
a. 1.96
b. 2.00
c. 7.00
d. 11.90
e. 12.05
Chapter 12 Solutions
Chemistry In Context
Ch. 12.1 - Skill Building Finding Equilibrium Glucose and...Ch. 12.1 - Prob. 12.3YTCh. 12.1 - Prob. 12.4YTCh. 12.2 - Prob. 12.5YTCh. 12.2 - Prob. 12.6YTCh. 12.2 - A practicing scientist must Judge a potential...Ch. 12.3 - Prob. 12.8YTCh. 12.3 - Skill Building Checking on Carbon a. Examine the...Ch. 12.3 - Prob. 12.10YTCh. 12.3 - Prob. 12.11YT
Ch. 12.3 - Prob. 12.12YTCh. 12.4 - Prob. 12.13YTCh. 12.4 - Skill Building Functional Groups in Dopamine Draw...Ch. 12.4 - Prob. 12.15YTCh. 12.5 - Prob. 12.16YTCh. 12.5 - Prob. 12.17YTCh. 12.6 - Prob. 12.18YTCh. 12.6 - Prob. 12.19YTCh. 12.6 - The structures of proteins, such as the ones shown...Ch. 12.7 - Reconsider your work in past chapters. List three...Ch. 12.7 - Prob. 12.22YTCh. 12.7 - Prob. 12.23YTCh. 12.8 - Prob. 12.24YTCh. 12.8 - Prob. 12.25YTCh. 12.9 - Skill Building Ester Formation Draw structural...Ch. 12.9 - Prob. 12.27YTCh. 12.9 - You Decide Supersize My Aspirin A friend who...Ch. 12.9 - Modern methods of drug discovery involve...Ch. 12.10 - Make two lists of drugs for each of the two...Ch. 12.10 - See for yourself the shapes of drug molecules by...Ch. 12.10 - Prob. 12.33YTCh. 12.10 - Prob. 12.34YTCh. 12 - Scientific Practices Follow the Hormone Using the...Ch. 12 - The field of chemistry has many sub-disciplines....Ch. 12 - Prob. 2QCh. 12 - Prob. 4QCh. 12 - Nitrous acid (HNO2) has a Ka value of 4.0 10 4,...Ch. 12 - Use the Henderson-Hasselbalch equation and Table...Ch. 12 - Write the structural formula and line-angle...Ch. 12 - Prob. 8QCh. 12 - Prob. 9QCh. 12 - Prob. 10QCh. 12 - Prob. 11QCh. 12 - Prob. 12QCh. 12 - Estradiol is relatively insoluble in water but...Ch. 12 - Prob. 14QCh. 12 - Prob. 15QCh. 12 - Prob. 16QCh. 12 - Define and relate the two terms: hormone and...Ch. 12 - Refer to Figure 11.17. Select two examples of...Ch. 12 - Prob. 19QCh. 12 - Molecules as diverse as cholesterol, sex hormones,...Ch. 12 - Prob. 21QCh. 12 - Prob. 22QCh. 12 - Prob. 23QCh. 12 - Sulfanilamide is the simplest sulfa drug, a type...Ch. 12 - Explain why an equilibrium constant cannot tell...Ch. 12 - Use the information in Table 12.1 to redraw Figure...Ch. 12 - Draw structural formulas for each of these...Ch. 12 - In Your Turn 12.12, you were asked to draw...Ch. 12 - Prob. 29QCh. 12 - Prob. 30QCh. 12 - Prob. 31QCh. 12 - Prob. 32QCh. 12 - Prob. 34QCh. 12 - Prob. 35QCh. 12 - Prob. 36QCh. 12 - Prob. 37QCh. 12 - Prob. 38QCh. 12 - The text states that some racemic mixtures contain...Ch. 12 - Prob. 40QCh. 12 - Prob. 41QCh. 12 - Prob. 44QCh. 12 - Prob. 47QCh. 12 - Prob. 49QCh. 12 - Dorothy Crowfoot Hodgkin first determined the...Ch. 12 - Prob. 52Q
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A buffer solution contains dissolved C₆H₅NH₂ and C₆H₅NH₃Cl. The initial concentration of C₆H₅NH₂ is 0.50 M and the pH of the buffer is 4.20. Determine the concentration of C₆H₅NH₃⁺ in the solution. The value of Kb for C₆H₅NH₂ is 3.8 × 10⁻¹⁰. Let x equal the original concentration of C₆H₅NH₃⁺ in the water. Based on the values given, set up an ice table to determine the unknown. Based on the ice table, set up the expression of Kb in order to determine the unknown. Do not simplify or combine terms. Based on the ice table and kb expression, determine the original concentration of C₆H₅NH₃⁺.arrow_forwardHarriet Rowki prepared 100.0 mL of 2.50 M H3BO3-NaH2BO3 buffer solution (pH = 8.50). The pKa value of H3BO3 is 9.24. A. Write the net ionic equation describing the buffer system. B. By writing the chemical reactions involved, show how the buffer system resists the drastic change in pH after adding 1 drop 0.100 M HCl or 1 drop 0.100 M of NaOH.arrow_forwardA 0.3700 g sample of an unknown monoprotic acid is diluted to 50.00 mL with water. The solution is then titrated with a 0.1250 M NaOH solution, and 40.00 mL were required to reach the equivalence point. The pH of solution was 7.00 after 21.15 mL was added. Determine the molecular weight of the acid and its pKaarrow_forward
- Calculate the pH of a buffered solution that is prepared by dissolving 0.3mol of NaHCO3and 0.45mol of Na2CO3 in 1L of water at 298 K? (NaHCO3 pKa=10.27) 2. What change in pH would be obeserved upon the addition of 0.02 mol of HCl solution to (a) the buffered solution above and (b) 1L of pure water? (hint: which buffer component responds to the presence of the strong acid?)arrow_forwardWhat will be the appropriate pH of a buffer prepared by mixing 100 ml of 0.1 M Na2HPO4 and 100 ml of 0.1 M NaH2PO4? (For H3PO4, pKa1 = 2.1; pKa2 = 6.8; pKa3 = 12.5) Ans in 2 significant figuresarrow_forwardWhy is the pH of the first, second and third equivalence point, of a titration of triprotic acid and NaOH ? Pka 1= 3.128 Pka 2=4.761 Pka 3= 6.396 Concentration of acid : 0,026 M Volume of acid : 50 ml Concentration of NaOh = 99,986mMarrow_forward
- 1. What is the pH of a buffer that contains 1.100 M NH3 and 1.300 M NH4+? Ka= 5.70x10-10What would be the new pH if a 100 mL portion of 0.075 M HCl is added to another 400 mL portion of the buffer? 2. If a multivitamin was manufactured on March 2021, when is its most likely expiration date?a. June 2021b.December 2021c. September 2021d. March 2022arrow_forwardCalculate the pH of the solution after the addition of each of the given amounts of 0.0603 M HNO3 to a 60.0 mL solution of 0.0750 M aziridine. The pKa of aziridinium is 8.04. What is the pH of the solution after the addition of 70.5 mL HNO3? pH= What is the pH of the solution after the addition of a volume of HNO3 equal to the equivalence point?pH= What is the pH of the solution after the addition of 77.9 mL HNO3? pH=arrow_forwardYour goal is to make a buffer with a pH of 4.95 from acetic acid and sodium acetate. Assume the pKa for acetic acid is 4.74. What is the volume of acetic acid added to make your buffer? Report your answer to the tenths place. To do this, we use the Henderson-Hasselbach Equation pH = pkA + log (base/acid) where pkA = 4.74 for acetic acid, pH = target pH and [base] /[acid] = x/(1-x) since we don't know the concentration of the base, we will call it x the acid is just 1-x, assuming the two add up to 100% solve for x, and then you know the mL of base and acid to add.arrow_forward
- B) What is the pH at the equivalence point? C) Give the Ka and pKa value of the acid. Explain.arrow_forwardPhosphoric acid is a triprotic acid (?a1=6.9×10^3Ka1=6.9×10^3, ?a2=6.2×10^8 Ka2=6.2×10−8, and ?a3=4.8×10^13Ka3=4.8×10^13). Calculate the pH of a buffer solution obtained by dissolving 19.0 g of KH2PO4(s) and 30.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. pH=arrow_forwardA student wishes to make a buffer that has a pH of 4.65. The student has HNO2 and NaNO2. Nitrous Acid (HNO2) has a pKa of 3.40 The student makes 100.0 mL of a 0.100 M solution of HNO2. What mass (in grams) of NaNO2 should the student add to the solution to make a buffer that buffers at a pH of 4.25. (Hint: use the Henderson-Hasselbalch equation to find the molarity, then find the number of moles required to achieve that concentration in 100.0 mL of solution.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY