
Concept explainers
(a)
Interpretation:
Chiral carbon atom in naproxen and L-DOPA has to be identified.
Concept Introduction:
Chirality: It refers to an atom in a molecule that contains four different substituents.
(b)
Interpretation:
Concept Introduction:
Functional group: They are certain substitutes in the organic molecules which are determine the characteristic reactions taking place in it.
Alcohol: It is an organic compound where it contains at least one
Carboxylic acid: One
Ether: Ether is a group of organic compound where two aryl or alkyl groups are connected by an oxygen atom. It is represented as
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
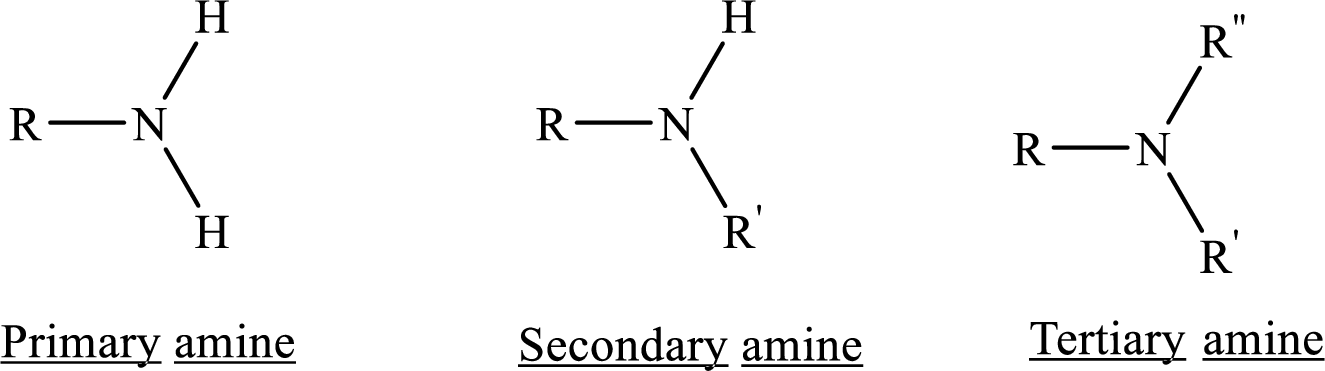
Aromatic Compounds: Compounds that are planar, cyclic and having
(c)
Interpretation:
Structural formula for the mirror images of naproxen and L-DOPA has to be identified.
Concept Introduction:
In chemistry, structure is the arrangement of
In structural formula,
- All of the atoms are shown at each end and intersection of bonds
- H atoms are shown
Enantiomers: The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Chemistry In Context
- Use the web to research the amount of PVC polymer produced annually in the United States. What are the three most common uses of this polymer?arrow_forwarddrawing of the complete Lewis structure for vitamin b5, showing all atoms, bonds, lone pairs, and charges (as applicable). This structure must be hand-drawn by your group, not a printout from a webpage. c) Identification of all of the organic functional groups for your molecule, clearly labeled on your drawing of its Lewis structurearrow_forwardWhat are the alternative natural materials can be used in cleaning the following: A. food stain on clothes B. kitchen sink C. bad smell/odor inside refrigerator why these alternative materials can be used as household cleaning material?arrow_forward
- What role do you think the government should have in GMOs and why?arrow_forward1.What are the alternative natural materials can be used in cleaning the following: food stain on clothes kitchen sink bad smell/odor inside refrigerator 2.Give your opinion why these alternative materials can be used as household cleaning materialarrow_forwardWhat type of chemical reaction is taking place (inside the banana) as it ripens? Illustrate using the structures of reactants and productsarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





