
(a)
Interpretation:
Similarities and differences in the structures of aspirin, ibuprofen and acetaminophen have to be identified.
Concept Introduction:
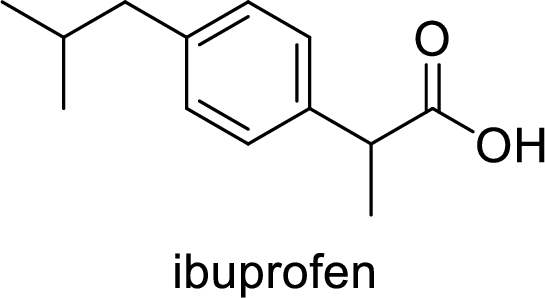
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) used for the treatment of fever and inflammation. Side effects commonly seen for ibuprofen are bloating, headache, nausea etc. The structure of ibuprofen is,
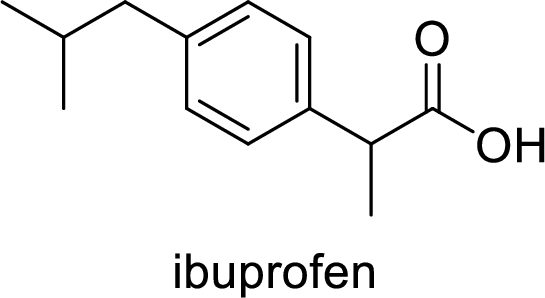
Acetaminophen used for the treatment of fever and pain. Side effects commonly seen for acetaminophen are jaundice, nausea, dark urine. The structure of acetaminophen is,
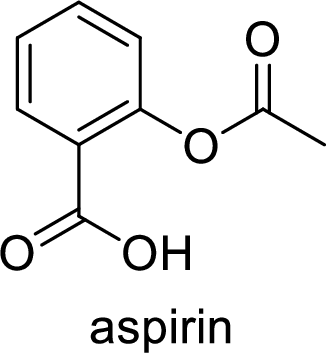
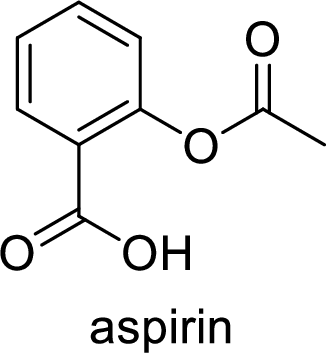
Aspirin used for the treatment of inflammation, fever and pain. Side effects commonly seen for aspirin are stomach ache, nausea and rash. The structure of aspirin is,
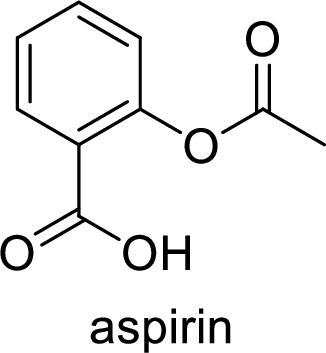
Alcohol: It is an organic compound where it contains at least one
(b)
Interpretation:
To find whether there is any similarities or differences in the symptoms that aspirin, ibuprofen and acetaminophen is promoted to treat have to be identified.
Concept Introduction:
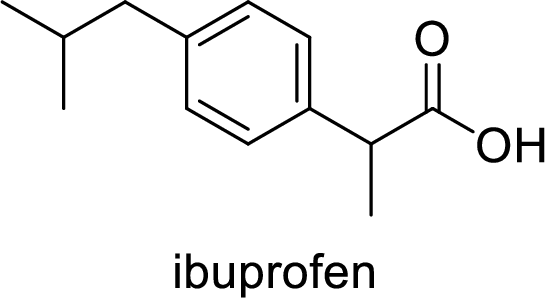
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) used for the treatment of fever and inflammation. Side effects commonly seen for ibuprofen are bloating, headache, nausea etc. The structure of ibuprofen is,
Acetaminophen used for the treatment of fever and pain. Side effects commonly seen for acetaminophen are jaundice, nausea, dark urine. The structure of acetaminophen is,
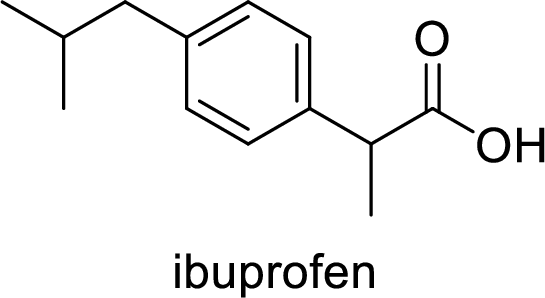
Aspirin used for the treatment of inflammation, fever and pain. Side effects commonly seen for aspirin are stomach ache, nausea and rash. The structure of aspirin is,
(b)
Interpretation:
To find whether there is any similarities or differences in the side effects of aspirin, ibuprofen and acetaminophen have to be identified.
Concept Introduction:
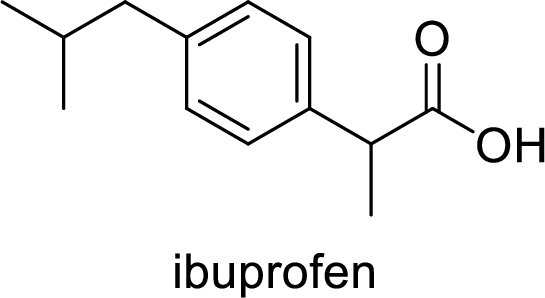
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) used for the treatment of fever and inflammation. Side effects commonly seen for ibuprofen are bloating, headache, nausea etc. The structure of ibuprofen is,
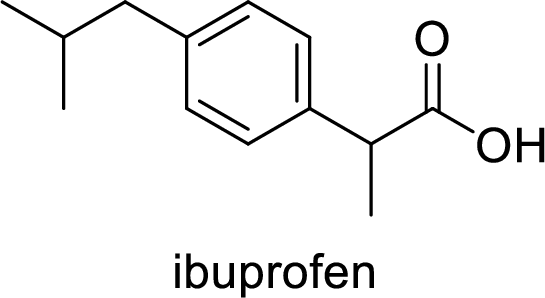
Acetaminophen used for the treatment of fever and pain. Side effects commonly seen for acetaminophen are jaundice, nausea, dark urine. The structure of acetaminophen is,
Aspirin used for the treatment of inflammation, fever and pain. Side effects commonly seen for aspirin are stomach ache, nausea and rash. The structure of aspirin is,
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Chemistry In Context
- no need for explanation just answerarrow_forwardI need expert solutions and step by step answerarrow_forwardTopic: Test of cation and anion.(qualitative) for each question right 4/5 bullet points 1. How this topic is related to Environmental science / bioinformatics? Choose 1 2. How this topic is related to our real life / industry? Choose one 3. Recent research and development of this topic. At least 4 bullet point each question, It will be very helpful for me .Thank you in advance .arrow_forward
- How can I solve this question? I need full details and accurate answer.arrow_forwardSo I went throught the material to re learn this question we had in class but I still don't get it. Maybe I don't understand my professors style of teaching as much. Can someone help me answer these questions and undertand them. Thank youarrow_forwardWrite a paraphrase of the following passages.1. Bats are in the limelight these days because they are rumored to be the source of COVID-19, the virus that caused the coronavirus pandemic. But that is just part of their story. Bats turn out to be miraculous creatures. Their ability to age without decrepitude or cancer, as well as fight off a multitude of infections, are giving us clues about how to do the same for ourselves.arrow_forward
- Hi! I do not have enough questions but I really need an enlightenment on these five questions. Thank youuu! It would help a lot.arrow_forwardI need help solving [635.968(Tf-291.5)]+[17.517(Tf-372.8)]=0. I would like the steps to get to the answer 293.7 so I can solve similar problems.arrow_forwardSolubility between Organic and Inorganic Compounds Procedure:1. Prepare 2 transparent glasses, tablespoon, water, table salt, and cooking or vegetable oil.2. Place water in both glasses and make sure it is 3⁄4 full. Take note, they should have the same volume.3. Add 1 tablespoonful of table salt on the first glass and stir it using the spoon for 3 minutes.4. Add 1 tablespoonful of oil on the second glass and stir it using the spoon for 3 minutes.5. Observe the results. From the procedure above (you can search),-Describe the solubility of table salt in water based from the experiment. -Describe the solubility of cooking or vegetable oil in water based from the experiment. -Which of the compounds used is an organic and an inorganic? -Aside from solubility, what are the other ways to differentiate organic and inorganic compounds? -Do all organic or inorganic compounds follow all their characteristic properties? Why or why not?arrow_forward
- Solubility between Organic and Inorganic Compounds Procedure:1. Prepare 2 transparent glasses, tablespoon, water, table salt, and cooking or vegetable oil.2. Place water in both glasses and make sure it is 3⁄4 full. Take note, they should have the same volume.3. Add 1 tablespoonful of table salt on the first glass and stir it using the spoon for 3 minutes.4. Add 1 tablespoonful of oil on the second glass and stir it using the spoon for 3 minutes.5. Observe the results. From the procedure above (you can search),-Describe the solubility of table salt in water based from the experiment. -Describe the solubility of cooking or vegetable oil in water based from the experiment. -Which of the compounds used is an organic and an inorganic? -Aside from solubility, what are the other ways to differentiate organic and inorganic compounds? -Do all organic or inorganic compounds follow all their characteristic properties? Why or why not?Own answer please and I would appreciate it if answers could…arrow_forwardAn amino acid contains by mass: 34.28% C, 6.71% H, 13.32% N, and 45.67% O. What is its empirical formula? Question 3 options: C3H7NO3 C2H5N2O3 C3H9N2O2arrow_forwardNeed help with these please God Give you long lifearrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
