
Concept explainers
(a)
Interpretation:
The resulting alkane should be identified after reacting the following compound with
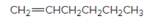
Concept Introduction:
Reaction of alkene with
Answer to Problem 53P
Explanation of Solution
Unsaturated (
(b)
Interpretation:
The resulting alkane should be identified after reacting the following compound with
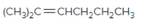
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 53P
Explanation of Solution
Unsaturated (
(c)
Interpretation:
The resulting alkane should be identified after reacting the following compound with
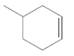
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 53P
Explanation of Solution
Unsaturated (
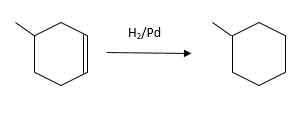
(d)
Interpretation:
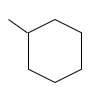
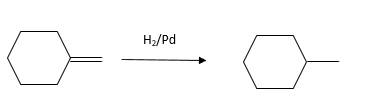
The resulting alkane should be identified after reacting the following compound with
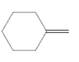
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 53P
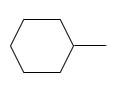
Explanation of Solution
Unsaturated (
Want to see more full solutions like this?
Chapter 13 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Draw the most stable conformation of pentane, using wedges and dashes to represent bonds coming out of the paper and going behind the paper, respectively.arrow_forward1. What is the alkane product from the reaction of C7H13COONa and NaOH? a. pentane b. hexane c. butane d. heptane 2. What is the resulting alkane if we have C5H11F and a C5H11F as reactants in Wurtz synthesis? a. hexane b. octane c. nonane d. decane 3.What is the resulting alkane if we have 2C2H5Cl as reactants in Wurtz Synthesis reaction? a. ethane b. butane c. hexane d. octanearrow_forwardCH3-CH2-OH reacts with H+/140C = ? + H2Oarrow_forward
- Name the alkene depicted in the ball-and-stick model, and draw the constitutional isomers formed when the alkene is treated with each reagent: (a) Br2; (b) Br2 in H2O; (c) Br2 in CH3OH.arrow_forwardIs the reaction of 2-butene with HBr regioselective? a. Is it stereoselective? b. Is it stereospecific?arrow_forward3. a. What is the chemical structure of benzoic acid, circle functional groups different than alkane,alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? __________________________arrow_forward
- Draw the products formed when cis- and trans-but-2-ene are treated with CHCl3 and KOC(CH3)3arrow_forwardExplain the solubility of alkanes, alkenes, and alkynes to H2SO4arrow_forwardDraw the Kekule Structure with lone pairs, the type of organic reaction (addition, elimination, substitution, and rearrangement reaction), and draw the move of electrons of the following: 1. H2C=CH2 + H2 --> CH3CH3arrow_forward
- As we learned in Chapter 4, monosubstituted cyclohexanes exist as an equilibrium mixture of two conformations having either an axial or equatorial substituent. When R = CH2CH3, Keq for this process is 23.When R = C(CH3)3, Keq for this process is 4000. a.When R = CH2CH3, which conformation is present in higher concentration? b.Which R shows the higher percentage of equatorial conformation at equilibrium? c. Which R shows the higher percentage of axial conformation at equilibrium? d. For which R is ΔGo more negative? e.How is the size of R related to the amount of axial and equatorial conformations at equilibrium?arrow_forwardDraw all the alkyl halides with molecular formula C 5H 11Cl formed when pentane (CH 3CH 2CH 2CH 2CH 3) is heated with Cl 2.arrow_forward1. Using the grignard reaction of alkanes what is the resulting alkane if the reactant is C4H9Br? a. ethane b. propane c. butane d. pentane 2. Using the grignard reaction of alkanes what is the resulting alkane if the reactant is C5H11F? a. ethane b. propane c. butane d. pentane 3. Using Cl2 in C2H4Cl2 will result in HCl and ______. a. C2H3Cl3 b. C2H4Cl3 c. C2H2Cl3 d. not posiblearrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





