
Concept explainers
(a)
Interpretation:
Skeletal structures of molecule A should be drawn by showing the stereochemistry at the carbon-carbon double bond.
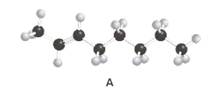
Concept Introduction:
The skeletal structure of a molecule is the formulaic representation excluding hydrogens.
(b)
Interpretation:
Stereoisomer of structure A should be drawn.
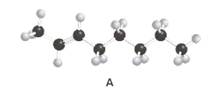
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Isomers are the different molecules that have the same molecular formula but differ in molecular arrangement in the space. There are several groups of isomers.
Stereoisomers are the form of isomers which have different three-dimensional orientations in the space.
(c)
Interpretation:
Constitutional isomer of structure A should be drawn.
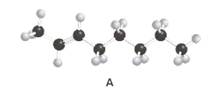
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Isomers are the different molecules that have the same molecular formula but differ in molecular arrangement in the space. There are several groups of isomers.
Constitutional isomers are the form of isomers which have atoms bonded to each other in different ways.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Draw the five constitutional isomers having molecular formula C6H14 ?arrow_forwardLabel following pairs of molecules as being either same same structure, completely different, constitutional isomers, or stereoisomers.arrow_forwarddraw two isomers with 4 carbon atoms in the main chain. be sure to inlcude all hydrogen atomsarrow_forward
- Based on the structures of lycopene and β-carotene, which are two chemicals found in another common vegetable, the tomato, answer the following questions: 1. Are they stereoisomers, constitutional isomers or not even isomers? 2. Do you expect them to be relatively polar or nonpolar substances? Why? 3. How many double bonds are in lycopene? How many in β -carotene? 4. As lycopene is transformed into β -carotene, how many pi electrons from the double bonds are transformed into single bonds? How many new single bonds are being formed in β -carotene? 5. The first step of a possible mechanism for the ring closure in the transformation is outlined below (only part of the structure is shown for simplicity). Draw the product that results from this arrow pushing mechanism. Be sure to include any formal charges that arise from the carbon atoms.arrow_forwardDraw a complete structure for vanillin showing all C atoms, H atoms, andlone pairs, and give the molecular formula. Vanillin is the principalcomponent of the extract of the vanilla bean. ?arrow_forwarda.Identify the functional groups in the ball-and-stick model of elemicin, a compound partly responsible for the flavor and fragrance of nutmeg. b. Draw a skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point. c. Label all electrophilic carbon atoms.arrow_forward
- a. Identify the functional groups in the ball-and-stick model of elemicin, a compound partly responsible for the flavor and fragrance of nutmeg. b. Draw a skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point. c. Label all electrophilic carbon atoms.arrow_forwardHow many stereogenic carbon/s is/are present in the molecule?arrow_forwardConsider the attached tricyclic structure B. (a) Label each substituent on the rings as axial or equatorial. (b) Draw B using chair conformations for each sixmembered ring. (c) Label the atoms on the ring fusions (the carbons that join each set of two rings together) as cis or trans to each other.arrow_forward
- Why is it not possible for a compound to have a quintary carbon (five groups attached to C)?arrow_forwardsort the compounds into pairs of constitutional isomers, stereoisomers, or identical moleculesarrow_forwardHello, I was reading an answer from the book Organic Chemistry by Brown et al. The question is 2.51 "Draw alternative chair conformations for each substituted cyclohexane and state which chair is more stable." There are then 4 cyclohexanes (labeled a-d). For the "a" cyclohexane, the substituents are arranged as a combination of axial and equatorial in both chair conformations. However, for the "b," "c," and "d," cyclohexanes one chair has only equatorial substituents, and the other chair has only axial substituents. Why is the "a" chair conformation different? https://www.bartleby.com/solution-answer/chapter-2-problem-251p-organic-chemistry-8th-edition/9781305580350/draw-alternative-chair-conformations-for-each-substituted-cyclohexane-and-state-which-chair-is-more/4532cd91-c341-11e9-8385-02ee952b546earrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
