
Concept explainers
(a)
Interpretation:
The given molecule is to be identified as
Concept introduction:
Answer to Problem 14.11P
The given molecule is nonaromatic.
Explanation of Solution
The structure of the given molecule is
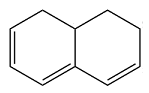
In the given compound, six carbon atoms are
Because of the
Therefore, this molecule is nonaromatic.
The presence of four
(b)
Interpretation:
The given molecule is to be identified as aromatic, antiaromatic, or nonaromatic.
Concept introduction:
Huckel’s rule for aromaticity states that if a species is planar and possesses a
Answer to Problem 14.11P
The given molecule is aromatic.
Explanation of Solution
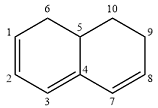
Structure of the given molecule is
In this molecule, six carbon atoms are
Therefore, the given molecule is aromatic.
The presence of six
(c)
Interpretation:
The given molecule is to be identified as aromatic, antiaromatic, or nonaromatic.
Concept introduction:
Huckel’s rule for aromaticity states that if a species is planar and possesses a
Answer to Problem 14.11P
The given molecule is nonaromatic.
Explanation of Solution
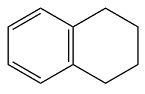
Structure of the given molecule is
In the given compound, four carbon atoms are
Therefore, the given molecule is nonaromatic.
The presence of one
(d)
Interpretation:
The given molecule is to be identified as aromatic, antiaromatic, or nonaromatic.
Concept introduction:
Huckel’s rule for aromaticity states that if a species is planar and possesses a
Answer to Problem 14.11P
The given molecule is nonaromatic.
Explanation of Solution
Structure of the given molecule is
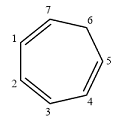
In the given compound, six carbon atoms are
Therefore, the given molecule is nonaromatic.
The presence of one
(e)
Interpretation:
The given molecule is to be identified as aromatic, antiaromatic, or nonaromatic.
Concept introduction:
Huckel’s rule for aromaticity states that if a species is planar and possesses a
Answer to Problem 14.11P
The given molecule is nonaromatic.
Explanation of Solution
Structure of the given molecule is
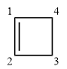
In the given compound, two carbon atoms are
Therefore, the given molecule is nonaromatic.
The presence of two
(f)
Interpretation:
The given molecule is to be identified as aromatic, antiaromatic, or nonaromatic.
Concept introduction:
Huckel’s rule for aromaticity states that if a species is planar and possesses a
Answer to Problem 14.11P
The given molecule is nonaromatic.
Explanation of Solution
Structure of the given molecule is
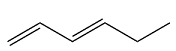
The molecule is not a cyclic system.
Therefore, the given molecule is nonaromatic.
Non-cyclic form of the given molecule indicates that the molecule is nonaromatic.
(g)
Interpretation:
The given molecule is to be identified as aromatic, antiaromatic, or nonaromatic.
Concept introduction:
Huckel’s rule for aromaticity states that if a species is planar and possesses a
Answer to Problem 14.11P
The given molecule is aromatic.
Explanation of Solution
The structure of the given molecule is
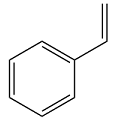
The molecule has a cyclic part with alternating single and double bonds. This means all ring atoms are
Therefore, this molecule is aromatic.
The presence of six
Want to see more full solutions like this?
Chapter 14 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Which are aromatic species? Please circle correct species. (assume planarity unless otherwise noted)arrow_forwardWhat is the hybridizarion of the atom labeled with arrow in each of the following molecules?arrow_forwardClassify each of the following molecules as a(n) aromatic, nonaromatic, or antiaromatic compound:arrow_forward
- please help with this question. thank you. Classify the following compounds (if flat) as aromatic, antiaromatic, or nonaromatic.arrow_forwardDetermine if the following is aromatic, antiaromatic or nonaromatic:arrow_forwardThese following structures are aromatic, antiaromatic or nonaromatic?arrow_forward
- Which of the molecules and ions given in diagram are aromatic according to the Hückel criteria? Which, if planar, would be antiaromatic?arrow_forwardPlease draw a more stable resonance structure for the following molecule. Use a curved arrow to show how to transform the original structure to the new one and please specify charges.arrow_forwardIn the first blank, label the molecule as either aromatic, antiaromatic, or nonaromatic. In the second, for aromatic and antiaromatic compounds provide the number of pi electrons that would be used. Place a zero in the second blank if non aromatic.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
