
Concept explainers
(a)
Interpretation:
The given compound is to be identified as
Concept introduction:
Heterocyclic compounds are defined as cyclic compounds which consist of at least one heteroatom, mostly nitrogen, sulfur, or oxygen. Heterocyclic compounds can be aromatic, antiaromatic, or nonaromatic. In some heterocyclic compounds, a noncarbon atom contributes to a p atomic orbital to the aromatic
Huckel’s
1) Aromatic if the number of electrons in that
Answer to Problem 14.20P
The given compound is antiaromatic.
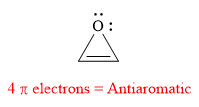
Explanation of Solution
The given compound is:
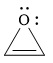
It is a heterocyclic compound with an oxygen atom. The hybridization of the oxygen atom must be

The total number of electrons in this
The given heterocyclic compound is antiaromatic if the total number of electrons in that
(b)
Interpretation:
The given compound is to be identified as aromatic, antiaromatic, or nonaromatic.
Concept introduction:
Heterocyclic compounds are defined as cyclic compounds which consists of at least one heteroatom mostly nitrogen, sulfur, or oxygen. Heterocyclic compounds can be aromatic, antiaromatic, or nonaromatic. In some heterocyclic compounds, a noncarbon atom contributes a p atomic orbital to the aromatic
Huckel’s rule for aromaticity states that if a species possesses a pi system of molecular orbitals constructed from p-orbitals that are fully conjugated around a ring (
1) Aromatic if the number of electrons in that
Answer to Problem 14.20P
The given compound is aromatic.
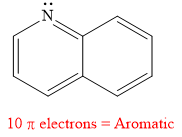
Explanation of Solution
The given compound is:
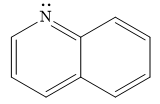
It is a heterocyclic compound with a nitrogen atom. The hybridization of the nitrogen atom must be

Thus, the total number of electrons in this
The given heterocyclic compound is aromatic if the number of electrons in that
(c)
Interpretation:
The given compound is to be identified as aromatic, antiaromatic, or nonaromatic.
Concept introduction:
Heterocyclic compounds are defined as cyclic compounds which consist of at least one heteroatom mostly nitrogen, sulfur, or oxygen. Heterocyclic compounds can be aromatic, antiaromatic, or nonaromatic. In some heterocyclic compounds, a noncarbon atom contributes a p atomic orbital to the aromatic
Huckel’s rule for aromaticity states that if a species possesses a pi system of molecular orbitals constructed from p-orbitals that are fully conjugated around a ring (
1) Aromatic if the number of electrons in that
Answer to Problem 14.20P
The given compound is nonaromatic.
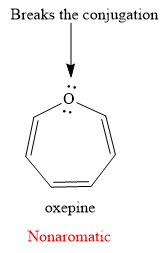
Explanation of Solution
The given compound is:
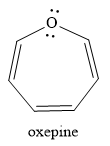
It is an oxygen containing a heterocycle consisting of a seven-membered ring with three double bonds. Due to geometrical constrains, the lone pairs of electrons on the oxygen atom are not in conjugation with the

If the molecule is assumed to be planar (flat), then too it would contain
The rule for aromaticity or antiaromaticity applies only if the system is planar, cyclic and has overlap of p-orbitals.
(d)
Interpretation:
The given compound is to be identified as aromatic, antiaromatic, or nonaromatic.
Concept introduction:
Heterocyclic compounds are defined as cyclic compounds which consists of at least one heteroatom mostly nitrogen, sulfur, or oxygen. Heterocyclic compounds can be aromatic, antiaromatic, or nonaromatic. In some heterocyclic compounds, a noncarbon atom contributes a p atomic orbital to the aromatic
Huckel’s rule for aromaticity states that if a species possesses a pi system of molecular orbitals constructed from p-orbitals that are fully conjugated around a ring (
1) Aromatic if the number of electrons in that
Answer to Problem 14.20P
The given compound is nonaromatic.
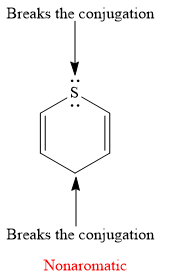
Explanation of Solution
The given compound is:
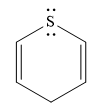
It is a sulfur containing a heterocycle consisting of a six-membered ring with two double bonds. There are two lone pair of electrons on the sulfur atom, but the sulfur atom is

As the
The rule for aromaticity or antiaromaticity applies only if the system is planar, cyclic and has overlap of p-orbitals.
(e)
Interpretation:
The given compound is to be identified as aromatic, antiaromatic, or nonaromatic.
Concept introduction:
Heterocyclic compounds are defined as cyclic compounds which consists of at least one heteroatom mostly nitrogen, sulfur, or oxygen. Heterocyclic compounds can be aromatic, antiaromatic, or nonaromatic. In some heterocyclic compounds, a noncarbon atom contributes a p atomic orbital to the aromatic
Huckel’s rule for aromaticity states that if a species possesses a pi system of molecular orbitals constructed from p-orbitals that are fully conjugated around a ring (
1) Aromatic if the number of electrons in that
Answer to Problem 14.20P
The given compound is nonaromatic.
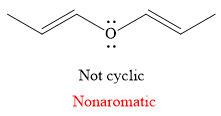
Explanation of Solution
The given compound is:
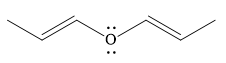
It is a straight chain compound with an oxygen atom and two double bonds. The above compound is not a cyclic compound. The rule for aromaticity or antiaromaticity applies only if the system is planar, cyclic and has overlap of p-orbitals. Thus, the

The rule for aromaticity or antiaromaticity applies only if the system is planar, cyclic and has overlap of p-orbitals.
(f)
Interpretation:
The given compound is to be identified as aromatic, antiaromatic, or nonaromatic.
Concept introduction:
Heterocyclic compounds are defined as cyclic compounds which consists of at least one heteroatom mostly nitrogen, sulfur, or oxygen. Heterocyclic compounds can be aromatic, antiaromatic, or nonaromatic. In some heterocyclic compounds, a noncarbon atom contributes a p atomic orbital to the aromatic
Huckel’s rule for aromaticity states that if a species possesses a pi system of molecular orbitals constructed from p-orbitals that are fully conjugated around a ring (
1) Aromatic if the number of electrons in that
Answer to Problem 14.20P
The given compound is aromatic.
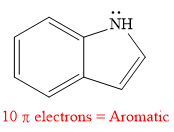
Explanation of Solution
The given compound is:
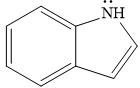
It is a heterocyclic compound with a nitrogen atom in which one six-membered and one five-membered rings are fused. The hybridization of the nitrogen atom is

Thus, the total number of electrons in this
The given heterocyclic compound is aromatic if the number of electrons in that
Want to see more full solutions like this?
Chapter 14 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Which are aromatic species? Please circle correct species. (assume planarity unless otherwise noted)arrow_forwardIn the first blank, label the molecule as either aromatic, antiaromatic, or nonaromatic. In the second, for aromatic and antiaromatic compounds provide the number of pi electrons that would be used. Place a zero in the second blank if non aromatic.arrow_forwardplease help with this question. thank you. Classify the following compounds (if flat) as aromatic, antiaromatic, or nonaromatic.arrow_forward
- Determine if the following is aromatic, antiaromatic or nonaromatic:arrow_forwardBased on your answer to below Problem, do you thinkthe compound shown here should have a significantdipole moment? If so, in which direction does it point? The molecule shown here has quite a large dipole, asindicated in its electrostatic potential map. Explain why.Hint: Consider various resonance structures.arrow_forwardThe molecule shown here has quite a large dipole, as indicated in its electrostatic potential map. Explain why.Hint: Consider various resonance structures.arrow_forward
- Classify each of the following molecules as a(n) aromatic, nonaromatic, or antiaromatic compound:arrow_forwardPlease draw a more stable resonance structure for the following molecule. Use a curved arrow to show how to transform the original structure to the new one and please specify charges.arrow_forwardDraw the resonance structure for the following molecule: Just started with resonance, where would the arrows go? I'm unsure of this problem.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
