
Concept explainers
(a)
Interpretation:
Whether the given molecule is
Concept introduction:
The molecules, to be aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic due to lack of planarity.
Explanation of Solution
The given structure is:
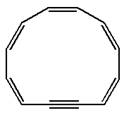
The molecule has total
The molecule is determined as nonaromatic based on the structure planarity.
(b)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic due to lack of planarity.
Explanation of Solution
The given structure is:
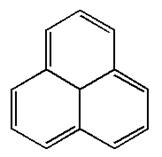
The molecule has total
The molecule is determined as nonaromatic based on the structure planarity.
(c)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is aromatic as it is planar, has cyclic conjugation, and obeys Hückel’s rule.
Explanation of Solution
The given structure is:
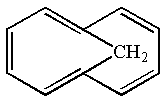
The given molecule is planar and has total
The molecule is determined as aromatic based on the Hückel’s rule.
(d)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic due non cyclic conjugated system.
Explanation of Solution
The given structure is:
![]()
The molecule has a conjugated system of total
The molecule is determined as nonaromatic based on the non cyclic structure which lacks cyclic conjugation.
(e)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is aromatic as it is planar, has cyclic conjugation, and obeys Hückel’s rule.
Explanation of Solution
The given structure is:
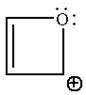
The molecule is planar and has fully cyclic conjugated system of total
The molecule is determined as aromatic based on planarity, fully conjugated system, and having Hückel’s rule of
(f)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic as it is not conjugated and does not obey Hückel’s rule.
Explanation of Solution
The given structure is:
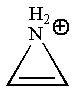
The molecule has only
The molecule is determined as nonaromatic based on the non-conjugated system of
(g)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic as it has no cyclic conjugated system.
Explanation of Solution
The given structure is:
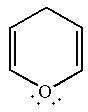
One of the lone pair on the oxygen atom participates in the resonance, thus, the molecule has
The molecule is determined as nonaromatic based on non-conjugated system of
Want to see more full solutions like this?
Chapter 14 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Which or which of the following molecules are aromatic, antiaromatic or non-aromatic?arrow_forwardwhich molecules should be aromaticarrow_forwardQuestion (Organic Chemistry) Consider molecules A–D. Does the arrow pushing in each structure leadto an acceptable resonance form? If so, explain why it is acceptable or unacceptable? What are the rules and logic? If so, draw it and carefully explain your answer and provide explanations for why each structure leds to an acceptable or unacceptable resonance form. Why is the arrow pushing acceptable or unacceptable in each individual case? Explain you answer with many details, logic, and in a step-by-step fashion.arrow_forward
- Question (Organic Chemistry) Consider molecules A–B. Does the arrow pushing in each structure leadto an acceptable resonance form? If so, explain why it is acceptable or unacceptable? What are the rules and logic? If so, draw it and carefully explain your answer and provide explanations for why each structure leds to an acceptable or unacceptable resonance form. Why is the arrow pushing acceptable or unacceptable in each individual case? Explain you answer with many details, logic, and in a step-by-step fashion. Please explain your rationale or logic. The answers provided on Bartleby lack a clear rationale and explanation of why the octet rule matters or why electron rich and electron deficient sites are important to this drawings.arrow_forwardUse Huckel’s rule to identify if its aromatic or not. Draw the resonance structure of the ff. compounds. note: I need the answer immediately. I will send a good rate right away as well.arrow_forwardare these aromatic, antiaromatic, or nonaromatic? Draw in all lonepairs and indicate the orbital each lone pair resides in.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
