
Concept explainers
(a)
Interpretation:
Relationship between two given Fischer projections by means of stereoisomerism (identical or enantiomers) should be determined.
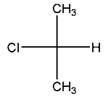 and
and 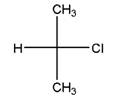
Concept Introduction:
Identical molecules are the ones with no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural connectivity (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
Fischer Projection is a method of drawing 3-D structures of organic molecules using cross formula. In this method, all non-terminal bonds are depicted as horizontal or vertical lines.
In the Fischer projection, horizontal bonds represent groups coming forward (drawn as wedges) and vertical bonds represent groups going backward (drawn as dashed wedges).
(b)
Interpretation:
Relationship between two given Fischer projections by means of stereoisomerism (identical or enantiomers) should be determined.
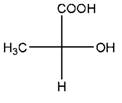 and
and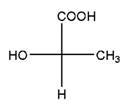
Concept Introduction:
Identical molecules are the ones with no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural connectivity (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
Fischer Projection is a method of drawing 3-D structures of organic molecules using cross formula. In this method, all non-terminal bonds are depicted as horizontal or vertical lines.
In the Fischer projection, horizontal bonds represent groups coming forward (drawn as wedges) and vertical bonds represent groups going backward (drawn as dashed wedges).
(c)
Interpretation:
Relationship between two given Fischer projections by means of stereoisomerism (identical or enantiomers) should be determined.
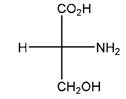 and
and 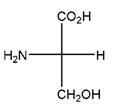
Concept Introduction:
Identical molecules are the ones with no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural connectivity (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
Fischer Projection is a method of drawing 3-D structures of organic molecules using cross formula. In this method, all non-terminal bonds are depicted as horizontal or vertical lines.
In the Fischer projection, horizontal bonds represent groups coming forward (drawn as wedges) and vertical bonds represent groups going backward (drawn as dashed wedges).
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Label each pair as enantiomer, diastereomer or same molecules.arrow_forwardConvert each three-dimensional representation into a Fischer projection.arrow_forwardFor those that have only one stereocenter draw Fischer projection(s) of the S-stereoisomer placing the 4 and 2 positions on the vertical line.arrow_forward
- Which of the following statements is correct regarding enantiomers? a. Enantiomers are constitutional isomers b. Enantiomers are meso compounds c. Enantiomers are mirror images to each other d. Enantiomers have only one chiral centerarrow_forwardDraw the Fischer projection for structure I. Circle each chiral group in structure II.arrow_forwardDraw all the possible stereoisomers for each compound and label pairs of enantiomers and diastereomers.arrow_forward
- How are the compounds in each pair related? Are they identical molecules or enantiomers?arrow_forwardLocate the stereogenic centers in each compound. A molecule may have one or more stereogenic centers. Gabapentin enacarbil [part (d)] is used to treat seizures and certain types of chronic pain.arrow_forwardWhich of the following is the definition of a pair of enantiomers? a. A pair of structures that are superposable mirror images of one another b. A pair of stereoisomers that are non-superposable mirror images of one another c. A pair of stereoisomers that are not mirror images of one another d. A pair of stereoisomers that have equal specific rotations Group of answer choices a b c darrow_forward
