
Concept explainers
(a)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the
Answer to Problem 22P
The name of the compound is 3,3-dimethylbutanal.
Explanation of Solution
The given molecular formula of the aldehyde is:
Here the numbering of the carbon chain will start from the right where -CHO group is present. It can be clearly seen that the longest carbon chain containing the aldehyde group consists of four carbon atoms and two methyl substituentsare attached to carbon number 3.
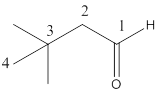
Therefore, the name of the compound becomes 3,3-dimethylbutanal.
(b)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al in the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon number 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
Answer to Problem 22P
The name of the compound is 4-ethylheaxanal.
Explanation of Solution
The given molecular formula of the aldehyde is:
Here the numbering of the carbon chain will start from the right where -CHO group is present. It can be clearly seen that the longest carbon chain containing aldehydic group consists of six carbon atoms and an ethyl substituent is attached to carbon number4.
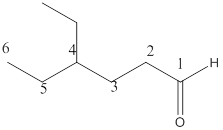
Therefore, the name of the compound becomes 4-ethylhexanal.
(c)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al in the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon no. 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
Answer to Problem 22P
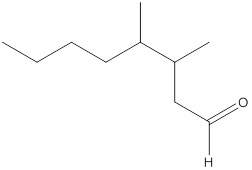
The name of the compound is 3,4-dimethyloctanal.
Explanation of Solution
The given molecular formula of the aldehyde is:
Here, the numbering of the carbon chain will start from the right where -CHO group is present. It can be clearly seen that the longest carbon chain containing the aldehyde group consists of eight carbon atoms and a methyl substituent is attached to carbon number 3 as well as carbon number 4.
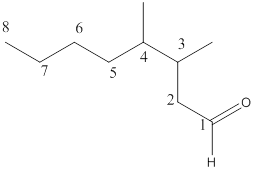
Therefore, the name of the compound becomes 3,4-dimethyloctanal.
(d)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al in the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon number 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
Answer to Problem 22P
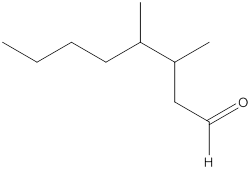
The name of the compound is 3-butylheptanal.
Explanation of Solution
The given molecular formula of the aldehyde can be written as follows:
Here, the numbering of the carbon chain will start from the right where -CHO group is present. It can be clearly seen that the longest carbon chain containing the aldehyde group consists of seven carbon atoms and abutyl substituent is attached to carbon number 3.
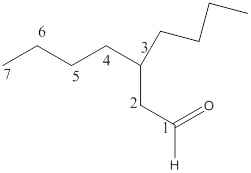
Therefore, the name of the compound becomes 3-butylheptanal.
(e)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al in the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon no. 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
Answer to Problem 22P
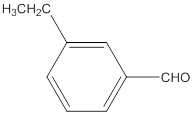
The name of the compound is 3-ethylbenzaldehyde.
Explanation of Solution
The given molecular formula of the aldehyde is:
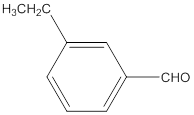
The name of the compound will be 3-ethylbenzaldehyde as the ethyl group is attached to the carbon number 3 of the parent molecule which is benzaldehyde. The benzene ring when attached to -CHO group is known as benzaldehyde.
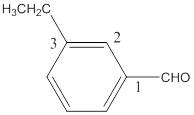
The naming of the molecule will start from the carbon to which aldehydic group is attached.
Want to see more full solutions like this?
Chapter 16 Solutions
General, Organic, and Biological Chemistry - 4th edition
- ___ is an example of an alkyl halide. Select one: a. KCl b. CHCl3 c. NaCl d. CF2=CF2arrow_forwarda. What is the chemical structure of biphenyl, circle functional groupsdifferent than alkane, alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? __________________________arrow_forwardHCl (aq), Zn(Hg) Br2, FeBr3 NBS, light KMnO4, H3O+ Mg metal, ether KOH, EtOH, heat CH3Cl, AlCl3 Dilute H3O+ ClCO(CH2)2CH3, AlCl3 NaCCCH2CH3 HNO3, H2SO4 2-butanonearrow_forward
- 1. What functional group is produced when an aldehyde reacts with H2/Pt? A.secondary alcohol B. carboxylic acid C.hemiacetal D. primary alcohol E.alkane F.tertiary alcohol G. alkene 2. What reaction occurs when an aldehyde reacts with H2/Pt to form a primary alcohol? A. Hydration B. Hydration C. Dehydration D. Oxidation E. Reduction( hydrogentation) 3. What reaction occurs when an Ester react with H+/H2O to from a carboxylic acid and alcohol? A. Dehydration B. Reduction ( Hydrogenation) C.Hydrolysis D. Hydration E.oxidationarrow_forwardMolecule Type Boiling point (°C) CH3CH2CH3 Alkane -42 CH3CHO Aldehyde +21 CH3CH2OH Alcohol +78 i. Why is the boiling point of the aldehyde greater than that of the alkane?ii. Why is the boiling point of alcohol the highest?iii. Explain why the solubility of aldehydes and alcohols falls as the molecules get bigger.arrow_forwardALCOHOLS 1. WHY IS ETHANOL MORE SOLUBLE IN WATER THAN 1-HEXANOL? 2. WHAT IS DENATURED ALCOHOL? AND WHY IS ALCOHOL DENATURED? ETHER 1. WHY DOES DIETHYL ETHER HAVE MUCH LOWER BOILING POINT THAN 1-BUTANOL?arrow_forward
- How to draw 2 propanolarrow_forwardGive three reasons why ethers make good laboratory reagentsarrow_forwardi. Why is the boiling point of the aldehyde greater than that of the alkane? ii. Why is the boiling point of alcohol the highest? iii. Explain why the solubility of aldehydes and alcohols falls as the molecules get bigger.arrow_forward
- n-Butyl methyl ether is an isomer of MTBE and has a boiling point of 70 oC. Explain why the boiling point is significantly different compared to MTBE.arrow_forwardWhat kind of solvent ingredients is usually used in the concentrations of 4-10 percent in skin care products and their function is to soften skin cells and to lessen wrinkles? A. Ethly acetate B. Alpha hydroxyl acids C. Phenols and phenol derivatives D. Aliphatic alcoholsarrow_forwardDraw an isomer of C5H10O that contains an etherarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
