
(a)
Interpretation:
The name of the following aldehyde is to be determined.
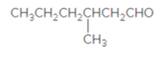
Concept Introduction:
While naming the
(b)
Interpretation:
The name of the following aldehyde is to be determined.
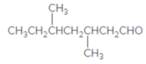
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al at the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon no. 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
(c)
Interpretation:
The name of the following aldehyde is to be determined.
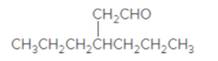
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al at the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon no. 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
(d)
Interpretation:
The name of the following aldehyde is to be determined.
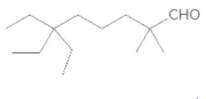
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al at the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon no. 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
(e)
Interpretation:
The name of the following aldehyde is to be determined.
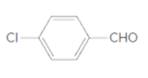
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al in the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon no. 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Draw the organic products for A and B.arrow_forwardWhat product is formed when a solution of A and B is treated with mild base? This reaction is the rst step in the synthesis of rosuvastatin (sold as a calcium salt under the trade name Crestor), a drug used to treat patients with high cholesterol.arrow_forwardDraw a stepwise mechanism for the following hydrolysis.arrow_forward
- (a) Give an acceptable name for compound A. (b) Draw the organic products formed when A is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forwardNot all aldehyde give a positve Bendicts test. Which of the follwing aldehydes do? a. d. b. e. c.arrow_forwardComplete these reactions. (a) (b)arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

