
Chemistry
9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 41E
Predict the sign of ∆S° for each of the following changes. Assume all equations are balanced.
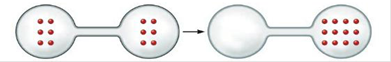
a.
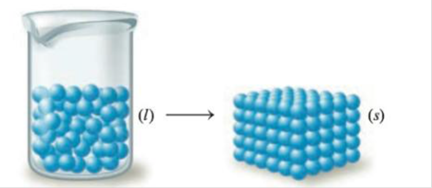
b.
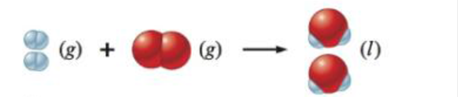
c.
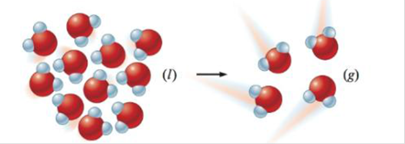
d.
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 17 Solutions
Chemistry
Ch. 17 - Define the following: a. spontaneous process b....Ch. 17 - What is the second law of thermodynamics? For any...Ch. 17 - What determines Ssurr for a process? To calculate...Ch. 17 - The free energy change, G, for a process at...Ch. 17 - What is the third law of thermodynamics? What are...Ch. 17 - What is the standard free energy change, G, for a...Ch. 17 - If you calculate a value for G for a reaction...Ch. 17 - Consider the equation G = G + RT ln(Q). What is...Ch. 17 - Even if G is negative, the reaction may not occur....Ch. 17 - Discuss the relationship between wmax and the...
Ch. 17 - For the process A(l) A(g), which direction is...Ch. 17 - For a liquid, which would you expect to be larger,...Ch. 17 - Gas A2 reacts with gas B2 to form gas AB at a...Ch. 17 - What types of experiments can be carried out to...Ch. 17 - A friend tells you, Free energy G and pressure P...Ch. 17 - Prob. 6ALQCh. 17 - Predict the sign of S for each of the following...Ch. 17 - Is Ssurr favorable or unfavorable for exothermic...Ch. 17 - At 1 atm, liquid water is heated above 100C. For...Ch. 17 - When (if ever) are high temperatures unfavorable...Ch. 17 - The synthesis of glucose directly from CO2 and H2O...Ch. 17 - When the environment is contaminated by a toxic or...Ch. 17 - Entropy has been described as times arrow....Ch. 17 - Human DNA contains almost twice as much...Ch. 17 - A mixture of hydrogen gas and chlorine gas remains...Ch. 17 - Consider the following potential energy plots: a....Ch. 17 - Ssurr is sometimes called the energy disorder...Ch. 17 - Given the following illustration, what can be said...Ch. 17 - The third law of thermodynamics states that the...Ch. 17 - The deciding factor on why HF is a weak acid and...Ch. 17 - List three different ways to calculate the...Ch. 17 - What information can be determined from G for a...Ch. 17 - Monochloroethane (C2H5Cl) can be produced by the...Ch. 17 - At 1500 K, the process I2(g)2I(g)10atm10atm is not...Ch. 17 - Which of the following processes are spontaneous?...Ch. 17 - Which of the following processes are spontaneous?...Ch. 17 - Table 16-1 shows the possible arrangements of four...Ch. 17 - Consider the following illustration of six...Ch. 17 - Consider the following energy levels, each capable...Ch. 17 - Redo Exercise 29 with two particles A and B, which...Ch. 17 - Choose the substance with the larger positional...Ch. 17 - Which of the following involve an increase in the...Ch. 17 - Predict the sign of Ssurr for the following...Ch. 17 - Calculate Ssurr for the following reactions at 25C...Ch. 17 - Given the values of H and S, which of the...Ch. 17 - At what temperatures will the following processes...Ch. 17 - Ethanethiol (C2H5SH; also called ethyl mercaptan)...Ch. 17 - For mercury, the enthalpy of vaporization is 58.51...Ch. 17 - For ammonia (NH3), the enthalpy of fusion is 5.65...Ch. 17 - The enthalpy of vaporization of ethanol is 38.7...Ch. 17 - Predict the sign of S for each of the following...Ch. 17 - Predict the sign of S for each of the following...Ch. 17 - For each of the following pairs of substances,...Ch. 17 - For each of the following pairs, which substance...Ch. 17 - Predict the sign of S and then calculate S for...Ch. 17 - Predict the sign of S and then calculate S for...Ch. 17 - For the reaction C2H2(g)+4F2(g)2CF4(g)+H2(g) S is...Ch. 17 - For the reaction CS2(g)+3O2(g)CO2(g)+2SO2(g) S is...Ch. 17 - It is quite common for a solid to change from one...Ch. 17 - Two crystalline forms of white phosphorus are...Ch. 17 - Consider the reaction 2O(g)O2(g) a. Predict the...Ch. 17 - Hydrogen cyanide is produced industrially by the...Ch. 17 - From data in Appendix 4, calculate H, S, and G for...Ch. 17 - The major industrial use of hydrogen is in the...Ch. 17 - For the reaction at 298 K, 2NO2(g)N2O4(g) the...Ch. 17 - At 100C and 1.00 atm, H = 40.6 kJ/mol for the...Ch. 17 - Given the following data:...Ch. 17 - Given the following data:...Ch. 17 - For the reaction SF4(g)+F2(g)SF6(g) the value of G...Ch. 17 - The value of G for the reaction...Ch. 17 - Consider the reaction...Ch. 17 - Consider the reaction 2POCl3(g)2PCl3(g)+O2(g) a....Ch. 17 - Using data from Appendix 4, calculate H, S and G...Ch. 17 - Consider two reactions for the production of...Ch. 17 - Using data from Appendix 4, calculate G for the...Ch. 17 - Using data from Appendix 4, calculate G for the...Ch. 17 - Consider the reaction 2NO2(g)N2O4(g) For each of...Ch. 17 - Consider the following reaction:...Ch. 17 - One of the reactions that destroys ozone in the...Ch. 17 - Hydrogen sulfide can be removed from natural gas...Ch. 17 - Consider the following reaction at 25.0C:...Ch. 17 - The standard free energies of formation and the...Ch. 17 - Calculate G forH2O(g)+12O2(g)H2O2(g) at 600. K,...Ch. 17 - The Ostwald process for the commercial production...Ch. 17 - Cells use the hydrolysis of adenosine...Ch. 17 - One reaction that occurs in human metabolism is...Ch. 17 - Consider the following reaction at 800. K:...Ch. 17 - Consider the following reaction at 298 K:...Ch. 17 - Consider the relationship In(K)=HRT+SR The...Ch. 17 - The equilibrium constant K for the reaction...Ch. 17 - Using Appendix 4 and the following data, determine...Ch. 17 - Some water is placed in a coffee-cup calorimeter....Ch. 17 - Calculate the entropy change for the vaporization...Ch. 17 - As O2(l) is cooled at 1 atm, it freezes at 54.5 K...Ch. 17 - Consider the following reaction:...Ch. 17 - Using the following data, calculate the value of...Ch. 17 - Many biochemical reactions that occur in cells...Ch. 17 - Carbon monoxide is toxic because it bonds much...Ch. 17 - In the text, the equation G=G+RTIn(Q) was derived...Ch. 17 - Prob. 91AECh. 17 - Use the equation in Exercise 79 to determine H and...Ch. 17 - Prob. 93AECh. 17 - Consider the following diagram of free energy (G)...Ch. 17 - Prob. 95CWPCh. 17 - For rubidium Hvapo=69.0KJ/mol at 686C, its boiling...Ch. 17 - Given the thermodynamic data below, calculate S...Ch. 17 - Consider the reaction: H2S(g)+SO2(g)3S(g)+2H2O(l)...Ch. 17 - The following reaction occurs in pure water:...Ch. 17 - Prob. 100CWPCh. 17 - Consider the reaction: PCl3(g)+Cl2(g)PCl5(g) At...Ch. 17 - The equilibrium constant for a certain reaction...Ch. 17 - Consider two perfectly insulated vessels. Vessel 1...Ch. 17 - Liquid water at 25C is introduced into an...Ch. 17 - Using data from Appendix 4, calculate H, G, and K...Ch. 17 - Entropy can be calculated by a relationship...Ch. 17 - a. Using the free energy profile for a simple...Ch. 17 - Consider the reaction H2(g)+Br2(g)2HBr(g) where H...Ch. 17 - Consider the system A(g)B(g) at25C. a. Assuming...Ch. 17 - The equilibrium constant for a certain reaction...Ch. 17 - If wet silver carbonate is dried in a stream of...Ch. 17 - Carbon tetrachloride (CCl4) and benzene (C6H6)...Ch. 17 - Sodium chloride is added to water (at 25C) until...Ch. 17 - You have a 1.00-L sample of hot water (90.0C)...Ch. 17 - Consider a weak acid, HX. If a 0.10-M solution of...Ch. 17 - Some nonelectrolyte solute (molar mass = 142...Ch. 17 - For the equilibrium A(g)+2B(g)C(g) the initial...Ch. 17 - What is the pH of a 0. 125-M solution of the weak...Ch. 17 - Impure nickel, refined by smelting sulfide ores in...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach
Practice Problem ATTEMPT
Write the rate expressions for each of the following reactions:
(a)
(b)
(c)
Chemistry
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Determine the de Brogue wavelength of a. an electron moving at 1/10 the speed of light. b. a 400 g Frisbee movi...
Inorganic Chemistry
Consider a sample of ideal gas initially in a volume V at temperature T and pressure P. Does the entropy of thi...
General Chemistry: Principles and Modern Applications (11th Edition)
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (14th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the sign of S, if possible, for each of the following reactions. If you cannot predict the sign for any reaction, state why. a HCl(g)+NH3(g)NH4Cl(s) b N2(g)+3H2(g)2NH3(g) c 2SO2(g)+O2(g)2SO3(g) d CH3OH(l)+32O2(g)CO2(g)+2H2O(g)arrow_forwardCalculate the standard entropy change for the reaction CO2(g)+2H2O(l)CH4(g)+2O2(g) . What does the sign of S say about the spontaneity of this reaction?arrow_forwardEthanol burns in air or oxygen according to the equation C2H5OH(l)+3O2(g)2CO2(g)+3H2O(g) Predict the sign of S for this reaction.arrow_forward
- Define the following: a. spontaneous process b. entropy c. positional probability d. system e. surroundings f. universearrow_forwardCalculate H and G for the following reactions at 25C, using thermodynamic data from Appendix C; interpret the signs of H and G. a Al2O3(s)+2Fe(s)Fe2O3(s)+2Al(s) b COCl2(g)+H2O(l)CO2(g)+2HCl(g)arrow_forwardWhen solid sodium acetate crystallizes from a supersaturated solution, can you accurately predict the sign of H for the crystallization? Why or why not?arrow_forward
- In the laboratory, POCl3 (phosphorus oxychloride) is used in the manufacture of phosphate esters, which are used in flame retardants and pesticides. It can be prepared by the following reaction at 25C: 2PCl3(g)+O2(g)2POCl3(g) H=572kJ;G=518.7kJ(a) Calculate S. Is the sign reasonable? (b) Calculate S for POCl3. (c) Calculate for POCl3.arrow_forwardConsider the reaction of 2 mol H2(g) at 25C and 1 atm with 1 mol O2(g) at the same temperature and pressure to produce liquid water at these conditions. If this reaction is run in a controlled way to generate work, what is the maximum useful work that can be obtained? How much entropy is produced in this case?arrow_forwardSodium carbonate, also called washing soda, can be made by heating sodium hydrogen carbonate: 2NaHCO3(l)Na2CO3(s)+CO2(g)+H2O(l) H=+135.6kJ;G=+34.6kJ at 25C (a) Calculate S for this reaction. Is the sign reasonable? (b) Calculate G at 0 K; at 1000 K.arrow_forward
- The free energy for a reaction decreases as temperature increases. Explain how this observation is used to determine the sign of either H or S.arrow_forwardConsider the reaction of 1 mol H2(g) at 25C and 1 atm with 1 mol Br2(l) at the same temperature and pressure to produce gaseous HBr at these conditions. If this reaction is run in a controlled way to generate work, what is the maximum useful work that can be obtained? How much entropy is produced in this case?arrow_forwardConsider the reaction 4NH3(g)+5O2(g)4NO(g)+6H2O(g) (a) Calculate ?H° for this reaction. Is it exothermic or endothermic? (b) Would you expect ?S° to be positive or negative? Calculate ?S°. (c) Is the reaction spontaneous at 25°C and 1 atm? (d) At what temperature, if any, is the reaction at equilibrium at 1 atm pressure?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax  General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY