
(a)
Interpretation:
Synthesis of
Concept introduction:
Synthesis is the process of conversion of one compound into another using reagents. For the conversion of one compound into another, multiple number of steps undergoes using multiple reagents.
Answer to Problem 20.47AP
Synthesis of
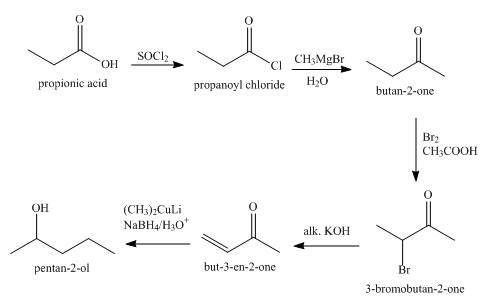
Explanation of Solution
The synthesis of

Figure 1
The total number of steps in the synthesis are five. In the first step propanoic acid is converted into acid chloride which is converted into
The ketone
Synthesis of
(b)
Interpretation:
Synthesis of the given compound from allyl alcohol using appropriate reagents is to be stated.
Concept introduction:
Synthesis is the process of conversion of one compound into another using reagents. For the conversion of one compound into another, the multiple numbers of steps undergo using multiple reagents.
Answer to Problem 20.47AP
Synthesis of the given compound from allyl alcohol using appropriate reagents is shown below.
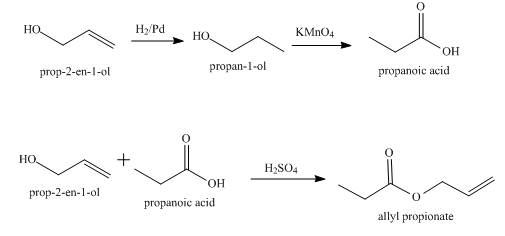
Explanation of Solution
The given compound to be synthesized is shown below.
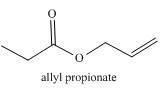
Figure 2
The synthesis of the given compound from allyl alcohol is done using multiple steps shown below.

Figure 3
The allyl alcohol,
Synthesis of the given compound from allyl alcohol using appropriate reagents is shown in Figure 3.
(c)
Interpretation:
Synthesis of
Concept introduction:
Synthesis is the process of conversion of one compound into another using reagents. For the conversion of one compound into another, the multiple numbers of steps undergo using multiple reagents.
Answer to Problem 20.47AP
Synthesis of
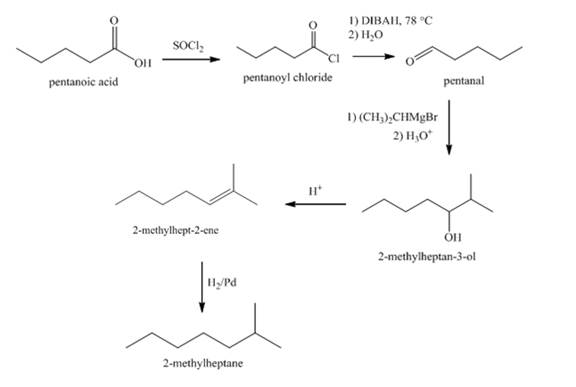
Explanation of Solution
The synthesis of

Figure 4
The conversion of pentanoic acid to acid chloride is done by using thionyl chloride. The acid chloride thus produced is reacted with DIBAH at
Synthesis of
(d)
Interpretation:
Synthesis of
Concept introduction:
Synthesis is the process of conversion of one compound into another using reagents. For the conversion of one compound into another, the multiple numbers of steps undergo using multiple reagents.
Answer to Problem 20.47AP
Synthesis of
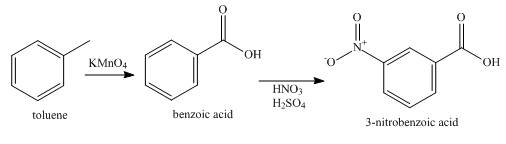
Explanation of Solution
The synthesis of

Figure 5
Toluene is first oxidized using potassium permanganate to give benzoic acid. A carboxylic acid group is a meta-directing group. The benzoic acid is then nitrated to give
Synthesis of
(e)
Interpretation:
Synthesis of
Concept introduction:
Synthesis is the process of conversion of one compound into another using reagents. For the conversion of one compound into another, the multiple numbers of steps undergo using multiple reagents.
Answer to Problem 20.47AP
Synthesis of
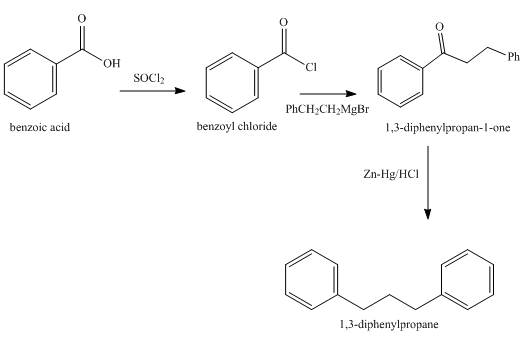
Explanation of Solution
The synthesis of

Figure 6
Benzoic acid is converted into benzoyl chloride in the first step which is then reacted with Grignard’s reagent to give an appropriate ketone which on Clemmensen reduction gives the required product
Synthesis of
(f)
Interpretation:
Synthesis of given
Concept introduction:
Synthesis is the process of conversion of one compound into another using reagents. For the conversion of one compound into another, the multiple numbers of steps undergo using multiple reagents.
Answer to Problem 20.47AP
Synthesis of given
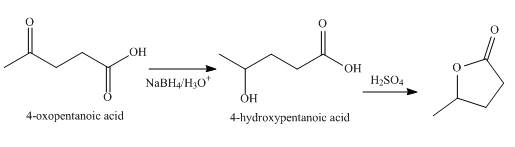
Explanation of Solution
The synthesized compound is
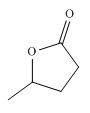
Figure 7
Synthesis of given

Figure 8
The
Synthesis of given
(g)
Interpretation:
Synthesis of given dicarboxylic acid from norbornene using appropriate reagents is to be stated.
Concept introduction:
Synthesis is the process of conversion of one compound into another using reagents. For the conversion of one compound into another, the multiple numbers of steps undergo using multiple reagents.
Answer to Problem 20.47AP
Synthesis of given dicarboxylic acid from norbornene using appropriate reagents is shown below.
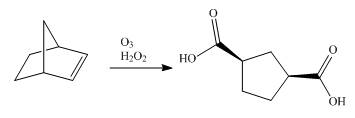
Explanation of Solution
The given dicarboxylic acid is shown below.
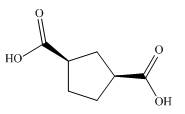
Figure 9
Synthesis of given dicarboxylic acid from norbornene using appropriate reagents is done in a single step shown below.

Figure 10
The conversion of norbornene into the required dicarboxylic acid is done in a single reaction. The ozonolysis of norbornene with oxidative work gives the required product.
Synthesis of given dicarboxylic acid from norbornene using appropriate reagents is shown in Figure 10.
(h)
Interpretation:
Synthesis of
Concept introduction:
Synthesis is the process of conversion of one compound into another using reagents. For the conversion of one compound into another, the multiple numbers of steps undergo using multiple reagents.
Answer to Problem 20.47AP
Synthesis of
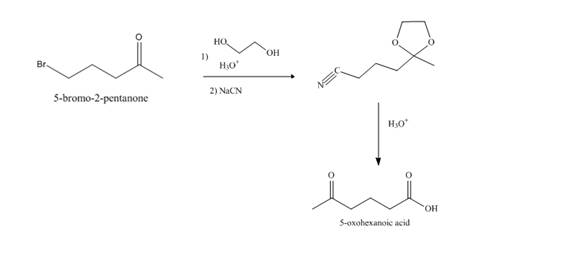
Explanation of Solution
Synthesis of

Figure 11
The compound,
Synthesis of
(i)
Interpretation:
Synthesis of
Concept introduction:
Synthesis is the process of conversion of one compound into another using reagents. For the conversion of one compound into another, the multiple numbers of steps undergo using multiple reagents.
Answer to Problem 20.47AP
Synthesis of
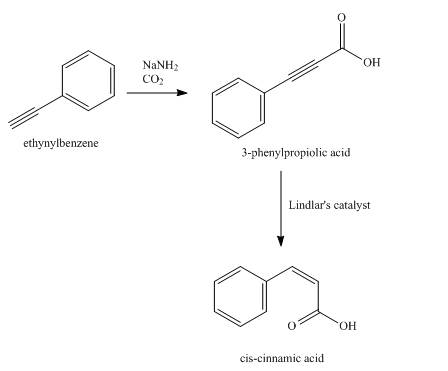
Explanation of Solution
Synthesis of

Figure 12
The compound,
Synthesis of
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- The pyrolysis of acetic esters to give an alkene and acetic acid is thought to involve a planar transition state and cyclic redistribution of (4n + 2) electrons. Propose a mechanism for pyrolysis of the following ester.arrow_forwardSuggest a reasonable series of reagents and reactions conditions to prepare cis-2-methylcyclohexly acetate (A) from trans-2-methylcyclohexanol.arrow_forwardPropose a synthesis to convert 1-methylcyclopentene B to the cyclic ester C. Show all intermediates, and reagents necessary for each step.arrow_forward
- An alkene with molecular formula C8H16 was obtained from ozonolysisreaction and give acetone and 2,2-dimethylpropanal as the product. Writethe chemical equation from the reactionarrow_forwardUsing the necessary inorganic reagents, propose the synthesis for 2,5-diphenylfuran from benzene and succinic acid.arrow_forwardA hydrocarbon (X), with the molecular formula: C8H14 is reduced in presence of sodium and liquid ammonia to give the only product (Y) with the molecular formula: C8H16. Compounds X and Y both resulting 2,5-dimethylhexane when treated with hydrogen and platinum catalyst (H2/Pt). As a result of the oxidative cleavage of compound Y (by using KMnO4 / H2SO4), a single carboxylic acid derivative with C4H8O2 molecular formula is formed. Again, as a result of the reaction of Y with perbenzoic acid, the chiral compound C8H14O is observed, but the reaction of compound Y with bromine gives the achiral C8H14Br2 as the product.arrow_forward
- Outline the synthesis of racemic 3-methyl-3-heptanol, Et(Me)C(*)OHn-Bu, starting from alcohols of four carbons or fewer and any inorganic reagents or solvent needed.arrow_forwardAn organic compound A which has a characteristic odour is treated with 50% NaOH to give B (C7H8O)and C which is a sodium salt of an organic acid . Oxidation of B gives back A. Heating C with soda lime yields an aromatic hydrocarbon D . Deduce the structures of A,B,C and Darrow_forwardTwo moles of organic compound ‘A’ on treatment with a strong base gives two compounds ‘B’ and ‘C’. Compound ‘B’ on dehydrogenation with Cu gives ‘A’ while acidification of ‘C’ yields carboxylic acid ‘D’ with molecular formula of CH2O2. Identify the compounds A, B, C and D and write all chemical reactions involved.arrow_forward
- Propose a synthesis for the following compounds. As starting materials, you may use cyclopentanol, alcohols containing no more than four carbon atoms, and any reagents and solvents. Trans-cyclopentane-1,2-diol.arrow_forwardA synthetic organic molecule, G, which contains both aldehyde and ether functional groups, is subjected to a series of reactions in a multi-step synthesis pathway. In the first step, G undergoes a Wittig reaction, leading to the formation of an alkene, H. Subsequently, H is treated with an ozone (O3) reagent followed by a reducing agent in an ozonolysis reaction, resulting in the formation of two different products, I and J. Considering the functional groups present in G and the nature of the reactions involved, what are the most probable structures or functional groups present in products I and J? A. I contains a carboxylic acid group, and J contains an aldehyde group. B. I contains a ketone group, and J contains an alcohol group. C. I and J both contain aldehyde groups. D. I contains an ester group, and J contains a ketone group. Don't use chat gpt.arrow_forwardPropose a synthesis for the following compound starting with benzene. Write out the reagents and intermediates of each step. (Assume ortho and para isomers can be seperated)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
