
(a)
Interpretation:
The name of the given compound is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.2P
The name of the given compound is
Explanation of Solution
The structure of the given compound is shown below.
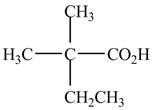
Figure 1
When a compound is named, the first thing is to identify the longest parent chain. If the parent chain is substituted, then it is numbered in such a manner so that the substituent gets the lowest locant number. In the given case, the structure is numbered as shown below.
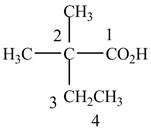
Figure 2
It contains a carboxyl group which is a higher priority functional group. The numbering is started from the carboxyl group. The parent chain is identified as butane. It is disubstituted at
The given compound is named as
(b)
Interpretation:
The name of the given compound is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.2P
The name of the given compound is
Explanation of Solution
The structure of the given compound is shown as below.
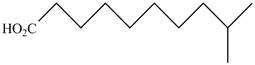
Figure 3
When a compound is named the first thing is to identify the longest parent chain. If the parent chain is substituted, then it is numbered in such a manner so that the substituent gets the lowest locant number. In the given case, the structure is numbered as shown below.
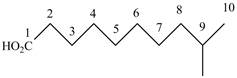
Figure 4
It contains a carboxyl group which is a higher priority functional group. The numbering is started from the carboxyl group. The parent chain is identified as decane. It is monosubstituted at
The given compound is named as
(c)
Interpretation:
The name of the given compound is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.2P
The name of the given compound is
Explanation of Solution
The structure of the given compound is shown as below.
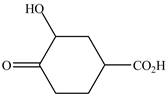
Figure 5
When a compound is named the first thing is to identify the longest parent chain. If the parent chain is substituted, then it is numbered in such a manner so that the substituent gets the lowest locant number. In the given case, the structure is numbered as shown below.
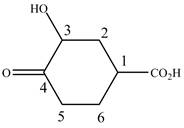
Figure 6
It contains a carboxyl group which is a higher priority functional group. The numbering is started from the carboxyl group. The parent chain is identified as cyclohexane. Cyclohexane is disubstituted at
The given compound is named as
(d)
Interpretation:
The name of the given compound is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.2P
The name of the given compound is
Explanation of Solution
The structure of the given compound is shown as below.
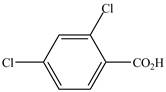
Figure 7
When a compound is named the first thing is to identify the longest parent chain. If the parent chain is substituted, then it is numbered in such a manner so that the substituent gets the lowest locant number. In the given case, the structure is numbered as shown below.
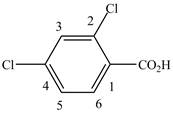
Figure 8
It contains a carboxyl group which is a higher priority functional group. The numbering is started from the carboxyl group. The parent chain is identified as benzene. Benzene is disubstituted at
The given compound is named as
(e)
Interpretation:
The name of the given compound is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.2P
The name of the given compound is
Explanation of Solution
The structure of the given compound is shown as below.
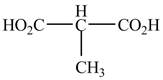
Figure 9
When a compound is named the first thing is to identify the longest parent chain. If the parent chain is substituted, then it is numbered in such a manner so that the substituent gets the lowest locant number. In the given case, the structure is numbered as shown below.
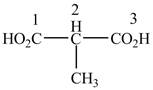
Figure 10
The numbering is started from the carboxyl group since the carboxyl group has higher priority over others. The parent chain is identified as propane. It contains two carboxyl groups named as a propanedioic acid. It is monosubstituted at
The given compound is named as
(f)
Interpretation:
The name of the given compound is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 20.2P
The name of the given compound is cyclopropanoic acid..
Explanation of Solution
The structure of the given compound is shown as below.
![]()
Figure 11
When a compound is named the first thing is to identify the longest parent chain. If the parent chain is substituted, then it is numbered in such a manner so that the substituent gets the lowest locant number. In the given case, the structure is numbered as shown below.
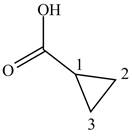
Figure 12
It contains a carboxyl group which is a higher priority functional group. The numbering is started from the carboxyl group. The parent chain is identified as cyclopropane. Therefore the given compound is named as cyclopropanoic acid
The given compound is named as cyclopropanoic acid.
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- (b) Classify the following alcohols in order of increasing ease of acid-catalyzed dehydration.Justify your answers.arrow_forwardProvide an acceptable name for the compound shown belowarrow_forwardCompound A is an alcohol that undergoes oxidation to produce compound B.Compound B is a ketone that gives positive triiodomethane reaction. Compound B isthen reacted with phenyl magnesium bromide, C6H5MgBr in the presence of aqueousacid to form compound C. Compound C has the molecular formula of C9H12O. Deducethe structure for compound A, B and C. PLEASE PROVIDE CLEAR DRAWINGS AND EXPLANATIONSarrow_forward
- Given the following compounds. Answer the following questions. Be sure to attach IR tables used as reference. Write the structure of your chosen answers. Provide clear explanation.arrow_forwardGive a clear handwritten answer with explanation needed of each compound !!!arrow_forwardWrite the structure of the Maleic Anhydride compound and label each non-equivalent carbon with a letter, A,B,C...arrow_forward
- Deduce the structure of compound C.arrow_forwardCompound X was soluble in water and ether, and its aqueous solution turned litmus blue. It reacted with sodium to give a gas. The compound reacted with benzenesulfonyl chloride and base to give an insoluble product, which was unchanged with acidification. It reacted with nitrous acid to give a yellow solid. Compound A could bearrow_forwardCan i get the answer answered with an explanation and example Why are 2-nitrobenzoic acid and 2-chlorobenzoic acid stronger acids than benzoic acid?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
